时间:2017-01-12 20:32:23
1、简答题 甲醇(CH3OH)和二甲醚(CH3OCH3)被称为21世纪的新型燃料,具有广泛的开发和应用前景.以CH4和H2O为原料制备二甲醚和甲醇的工业流程如下: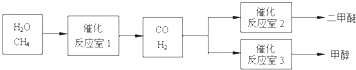
请填空:
(1)工业上一般采用下列两种反应成甲醇:
反应Ⅰ:CO(g)+2H2(g)═CH3OH(g)△H1
反应Ⅱ:CO2(g)+3H2(g)═CH3OH(g)+H2O(g)△H2
上述反应符合“原子经济”原则的是______(填“Ⅰ”或“Ⅱ”).
(2)在一定条件下,已知反应室2的可逆反应除生成二甲醚外还生成了气体CO2,其化学方程式为______.
(3)若利用水煤气合成二甲醚的三步反应如下:
①2H2(g)+CO(g)?CH3OH(g)△H=-90.8kJ?mol-1
②2CH3OH(g)?CH3OCH3(g)+H2O(g)△H=-23.5kJ?mol-1
③CO(g)+H2O(g)?CO2(g)+H2(g)△H=-41.3kJ?mol-1
则反应:3H2(g)+3CO(g)?CH3OCH3(g)+CO2(g)△H=______kJ/mol.
(4)某实验小组依据甲醇燃烧的反应原理,设计如图1所示的电池装置.则该电池正极的电极反应为:______.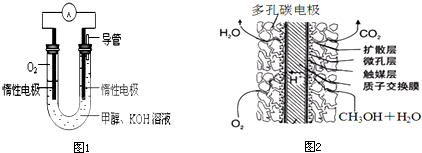
(5)甲醇质子交换膜燃料电池(如图2所示)是以酸性溶液为电解质溶液,甲醇从一个电极通入,O2从另一电极通入,中间为质子交换膜,通入甲醇的一极电极反应式为:______.
2、填空题 (1)化学反应可视为旧键断裂和新键形成的过程。化学键的键能是形成(或拆开)1 mol化学键时释放(或吸收)的能量。已知:N≡N键的键能是948.9kJ·mol-1,H-H键的键能是436.0 kJ·mol-1;由N2和H2合成1molNH3时可放出46.2kJ的热量。N-H键的键能是_________________
(2)盖斯定律在生产和科学研究中有很重要的意义。有些反应的反应热虽然无法直接测得,但可通过间接的方法测定。现根据下列3个热化学反应方程式:
Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) △H=-24.8 kJ·mol-1
3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g)△H=-47.2 kJ·mol-1
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) △H=+640.5 kJ·mol-1
写出CO气体还原FeO固体得到Fe 固体和CO2气体的热化学反应方程式:____________________________
3、选择题 甲醇质子交换膜燃料电池中将甲醇蒸气转化为氢气的两种反应原理是
①CH3OH(g)+H2O(g)?=?CO2(g)+3H2(g)??△H?=?+?49.0?kJ·mol-1
②CH3OH(g)+1/2O2(g)?=?CO2(g)+2H2(g)?△H?=-192.9?kJ·mol-1
下列说法正确的是??????????????????????????????????????? [???? ]
A.CH3OH的燃烧热为192.9?kJ·mol-1
B.反应①中的反应物总能量大于生成物的总能量
C.CH3OH转变成H2的过程一定要吸收能量???
D.根据②推知反应:CH3OH(l)+1/2O2(g)=CO2(g)+2H2(g)?的△H>-192.9?kJ·mol-1
4、选择题 298K时
Ca(s) + C(s,石墨)+ 3/2O2(g)= CaCO3(s) △H1=-1206.8kJ·mol-1 ①
Ca(s) + 1/2O2(g)= CaO(s) △H2=-635.1kJ·mol-1 ②
C(s,石墨)+ O2(g)= CO2(g) △H3=-393.5 kJ·mol-1 ③
计算CaCO3(s) = CaO(s) + CO2(g) △H4= ? [???? ]
A.+178.2 kJ·mol-1
B.-178.2 kJ·mol-1
C.+287.2 kJ·mol-1
D.-287.4 kJ·mol-1
5、选择题 已知:C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1;CO(g)+1/2O2(g) ===CO2(g) ΔH=-283 kJ·mol-1;则
C(s)与O2(g)反应生成1mol CO(g)的反应热为[???? ]
A.ΔH=-676.5 kJ·mol-1
B.ΔH=+676.5 kJ·mol-1
C.ΔH=-110.5 kJ·mol-1
D.ΔH=+110.5 kJ·mol-1