ʱ��:2021-02-18 04:56:03
1��ѡ���� 25��ʱ����20mL?0.1mol/L NaOH��Һ����μ���0.2mol/L������Һ��������ͼ��ʾ��
�й�����Ũ�ȹ�ϵ�ıȽ��У�����ȷ���ǣ�������
A����A�㣺c��Na+����c��OH-����c��CH3COO-����c��H+��
B����B�㣺c��OH-���Tc��H+����c��Na+���Tc��CH3COO-��
C����C�㣺c��CH3COO-����c��Na+����c��H+����c��OH-��
D����C�㣺c��CH3COO-��+c��CH3COOH���T2c��Na+��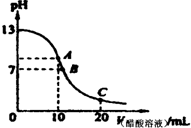
2��ѡ���� �����£���0.1mol?L-1����������Һ��0.06mol?L-1������Һ�������ϣ��û����Һ��pH���ڣ�������
A��1.7
B��2.0
C��12.0
D��12.4
3��ѡ���� ij�¶��£�CO2������Һ��Ũ����0.03 mol/L������1/3��CO2ת��ΪH2CO3����H2CO3����0.1%�������µ��룺H2CO3 H++ HCO3-������Һ��pHԼΪ
H++ HCO3-������Һ��pHԼΪ
[? ]
4��ѡ���� ��������pHΪ3��ij�ᣨHA����Һ��pHΪ11�İ�ˮ���������ϵû����Һ����������������Һ����Ե�������ȷ���ǣ�������
A����Һһ���Լ���
B����Һһ��������
C����Һ������������
D����Һ���������ԡ����Ի����
5��ѡ���� ��3����Һ��0.01mol?L-1���?��0.02mol?L-1������0.02mol?L-1NaOH�������Ϻ����Һ����0.04mol?L-1������0.02mol?L-1?NaOH�������Ϻ����Һ������˵������ȷ���ǣ�������
A��3����Һ��pH��С���Ǣ�
B���ں͢���Һ��������������ͬ
C��3����Һ��c��CH3COO-����С˳���Ǣۣ��ڣ���
D������������CH3COONa������
| c(CH3COO-) c(Na+) |