ʱ��:2017-11-11 01:01:32
1��ѡ���� ������Һһ�������Ե��ǣ�?��
A��c(H+)=10-7mol/L����Һ
B��pH=7����Һ
C��c(H+)/c(OH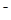 )=10-14����Һ
)=10-14����Һ
D����ˮ���Ȼ�淋Ļ��Һ��c(NH4+)=c(Cl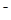 )
)
�ο��𰸣�D
���������
��ȷ�𰸣�D
A��Bֻ����25�棬KW=10�D14ʱ�����п��ܳ����ԣ�C��һ���ʼ��ԣ�D���ɵ���غ�c(H+)��c(NH4��)=c(OH�D )��c(Cl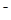 ), c(NH4+)=c(Cl
), c(NH4+)=c(Cl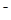 )ʱ��c(H+)=c(OH
)ʱ��c(H+)=c(OH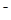 )
)
�����Ѷȣ���
2��ѡ���� ��֪��
Fe(s)+1/2O2(g)==FeO(s) ��H=-272 kJ��mol-1
2Al(s)+3/2O2(g)==Al2O3 (s) ��H=-1675. 8 kJ��mol-1
���������Ȼ�ѧ����ʽ���ж�����˵���в���ȷ����
[? ]
A������ȼ���ȡ�H=-1675.8 kJ��mol-1
B����������Fe(s)��Al(s)����������Ӧ�����ų���������
C��1 mol Al(s)������FeO(s)��ȫ��Ӧ���ų�429.9 kJ����
D��1 mol Al2O3 (s)�ֽ��Al(s)��O2(g)�Ĺ����У�������1675.8kJ����
�ο��𰸣�C
���������
�����Ѷȣ�һ��
3��ѡ���� �����Ȼ�ѧ����ʽ�С�H����ȼ���ȵ��ǣ�������
A��C6H12O6?��s��+6O2?��g��=6CO2?��g��+6H2O?��l����H1
B��S?��s��+
| 3 2 |
�ο��𰸣�A��������ȼ�����ɶ�����̼�����Һ̬ˮ���ų���������Ϊ�����ʵ�ȼ���ȣ���A��ȷ��
B������ȼ�����ɶ����������ų����ȼ�Ϊȼ���ȣ���B����
C������ȼ�����ɶ�����̼�����Һ̬ˮ���ų���������Ϊ�����ʵ�ȼ���ȣ���C����
D���Ȼ�ѧ����ʽ��ʾ����1mol��ȼ��ȼ�շų�����������D����
��ѡA��
���������
�����Ѷȣ�һ��
4��ѡ���� ��֪��HCN(aq)��NaOH(aq)��Ӧ����H="-12.1" kJ/mol��HCl(aq)��NaOH(aq)��Ӧ����H=-55.6kJ/mol����HCN��ˮ��Һ�е������H���ڣ�?��
A��-67.7 kJ/mol
B��-43.5 kJ/mol
C��+43.5 kJ/mol
D��+67.7 kJ/mol
�ο��𰸣�C
������������������غ㶨�ɿ�֪��HCN��ˮ��Һ�е������H��-12.1 kJ/mol��55.6kJ/mol��+43.5 kJ/mol����ѡC��
�����Ѷȣ�һ��
5��ѡ���� ����������ȷ���ǣ�?��
A��pH=3��pH=5�������10mL��ϣ�������Һ��pH=4
B����Һ��c��H+��Խ��pHֵҲԽ����Һ�����Ծ�Խǿ
C��Һ����Ȼ�����磬���ܽ���ˮ��������ã���ˣ�Һ��Ҳ��ǿ�����
D�����¶Ȳ���ʱ���ڴ�ˮ�м���ǿ����Һ����Ӱ��ˮ�����ӻ�����
�ο��𰸣�D
�����������
�����Ѷȣ���