ʱ��:2017-08-08 05:24:44
1��ѡ���� NaHA�Ƕ�Ԫ��H2A����ʽ�Σ�����˵����ȷ����
A����NaHA��ˮ��Һ�����ԣ����Ԫ��H2Aһ����ǿ��
B����NaHA��ˮ��Һ�ʼ��ԣ���HA��ֻ����ˮ��
C������H2A��ǿ�ỹ�����ᣬNaHA��Һ��Na����HA���ĸ���֮�ȶ���1�U1
D������H2A��ǿ�ỹ�����ᣬNaHA�����е��������Ӹ����ȶ���1�Ul
�ο��𰸣�D
���������A��һ����NaHA��ˮ��Һ�����ԣ�������˵�����β�ˮ�⡣�������ʽ�μȴ��ڵ���ƽ�⣬������ˮ��ƽ�⣬B����ȷ������H2A��ǿ�ỹ�����ᣬNaHA��Һ��Na����HA���ĸ���֮�ȶ�����1�U1��C����ȷ��������ȷ�Ĵ���D��
�����Ѷȣ���
2��ѡ���� ��1 L 0.3 mol��L��1��NaOH��Һ�л���ͨ��CO2��������Һ����8.8 g����������仯��������˵���в���ȷ����
A��������Һ�ʼ���
B��ȡ20mL������Һ��������ָʾ������0.2 mol��L��1��HCl ���еζ�������HCl�����Ϊ10mL
C��c(Na��) + c(H��) =" 2" c(CO32��) + c(HCO3��) + c(OH?��)
D��2c(Na��) = 3��c(CO32��) + c(HCO3��) + c(H2CO3)��
�ο��𰸣�B
�����������
�����Ѷȣ�һ��
3��ѡ���� ������Һ���й����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
A��NaHSO3��Һ�����ԣ����У�c��Na������c��HSO3-����c��SO32-����c��H������c��OH����
B��pH��ȵ�CH3COONa��Na2CO3������Һ��c��CH3COONa����c��Na2CO3��
C��ǿ��HA��Һ������MOH��Һ��Ϻ���Һ�����ԣ����У�c��M������c��A����
D��0.1 mol��L��1��NaHA��ҺpH��1�����У�c��Na������c��H2A����c��HA������c��A2����
�ο��𰸣�C
���������A�������������ӵ������������Ӻ�����������ӣ�ˮ�������ĵ������������Ӻ����������ӣ�����������Ũ�ȴ��������������Ũ�ȣ�A����B���ͬŨ�ȵ�CH3COONa��Na2CO3��Һ��CH3COO����ˮ��̶�С��CO32-��ˮ��̶ȣ�����pH��ͬ��CH3COONa��Na2CO3��Һ��c��CH3COONa����c��Na2CO3����C����ݵ���غ��c��M������c��H������c��A������c��OH������������Һ�����ԣ���c��H������c��OH����������c��M������c��A������D�0.1 mol��L��1��NaHA��ҺpH��1����NaHA=Na����H����A2����������Һ�в�����HA����H2A��
�����Ѷȣ�һ��
4��ѡ���� ��һ���¶��£�Na2CO3��Һ�д���ˮ��ƽ�⣺CO32-+H2O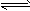 HCO3-+OH-������˵���У���ȷ����
HCO3-+OH-������˵���У���ȷ����
[? ]
A�������¶ȣ�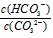 ��С
��С
B��ͨ��CO2��ƽ��������Ӧ�����ƶ�
C��ϡ����Һ��Na2CO3ˮ��̶�����ˮ��ƽ�ⳣ������
D������NaOH���壬ƽ�����淴Ӧ�����ƶ�����ҺpH��С
�ο��𰸣�B
���������
�����Ѷȣ�һ��
5������� ����13�֣�
(1)����2�֣����ʵ���Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ�� ��KNO3�� ��Na2CO3����NaHCO3����NaHSO4����CH3COOH����NaOH����NH4Cl��pH �ɴ�С��˳��Ϊ(�����)?
(2)����2�֣���AlCl3����ˮ���������������ɣ����գ�������ð�ɫ�������Ҫ�ɷ���?________??
(3)����������Һ�ܷ���ˮ��������ӷ���ʽ��ʾ�����ܷ���ˮ���д��������ˮ�⡱��������˵����Һ������ԣ�9�֣�
K 2CO3??��Һ��?��;
Na2SO4??��Һ��?��;
CuCl2?��Һ��?��;
�ο��𰸣�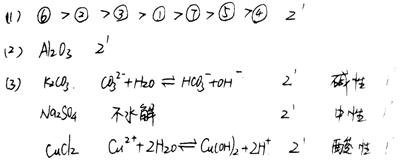
�����������
�����Ѷȣ���