ʱ��:2017-07-27 09:12:20
1��ѡ���� �ö��Ե缫���һ������Cu(NO3)2��Һ������˵���У���ȷ����? (? )
A�����������ĵ缫��ӦΪ��Cu2++2e��=Cu
B�����������ĵ缫��ӦΪ��4OH��=2H2O+O2��+4e�D
C������6.4 g����Cu�������ų���O2һ��Ϊ0.05 mol
D�������ɺ���һ������Cu(OH)2����Һ���ָܻ���ԭ����Ũ��
�ο��𰸣�D
���������
��ȷ�𰸣�D
A.����ȷ�����������ĵ缫��ӦΪ��Cu2++2e��=Cu?
B.����ȷ�����������ĵ缫��ӦΪ�� 2H2O�D4e�D=O2����4H��
C.����ȷ��ֻ����Cu(NO3)2����������£�����6.4 g����Cu�������ų���O2һ��Ϊ0.05 mol
D����ȷ����Cu (NO3)2���������ˮ��
�����Ѷȣ�һ��
2��ѡ���� �ö��Ե缫���ij����Ԫ��R��������R(NO3)n��Һ��ͨ��һ��ʱ���������������V L(��״��)ʱ��������������m g����R�����ԭ������ΪM����NAΪ�����ӵ�������ֵ,���������в���ȷ����
A����·��ͨ������ NA
NA
B��n �� 
C����Һ��H������ NA
NA
D��n �� 
�ο��𰸣�B
���������������4OH����4e�� 2H2O+O2��
2H2O+O2��  ?mol?
?mol? mol
mol
������Rn+?+?ne��? ?R
?R
n mol?M  ?mol?m g?n ��
?mol?m g?n ��  ?
?
���ʱ����ط�Ӧ����ʽΪ��
4R(NO3)n+2nH2O 4R+nO2��+4nHNO3
4R+nO2��+4nHNO3
e�� ?��? H+ ?mol?
?mol? ?mol
?mol
����B����ȷ��
�����Ѷȣ�һ��
3��ѡ���� Ӭ������Ĵ�����Ϣ�ؿ��ý���(����)������X��ŨNaOH��Һ�н������������õ�������ܷ�ӦʽΪ
C21H41COOH���ᣫX��4NaOH�D��C23H46��������Ϣ�أ���2Na2CO3��2H2O��H2��
������˵����ȷ����? (����)
A��XΪC3H7COOH
B������������ӦʽΪC21H41COOH��X��2e����2H2O=C23H46��CO32����6H��
C����������������pH����Na���������ƶ�
D�������Ļ�ԭ����ΪH2��OH��
�ο��𰸣�C
�������������ԭ���غ��֪X��ѧʽΪC3H6O2��A����Bѡ������CO32������һ����H�����ɣ�����H���������õ��ӿ�֪����H2�����Ե�������������pH����Cѡ����ȷ��OH�����ǻ�ԭ���Dѡ�����
�����Ѷȣ�һ��
4��ѡ���� Cu2O��һ�ְ뵼����ϣ�������ɫ��ѧ������Ƶ���ȡCu2O�ĵ���ʾ��ͼ���£�����ܷ�ӦΪ��
2Cu+H2O Cu2O+H2��������˵����ȷ����
Cu2O+H2��������˵����ȷ���� 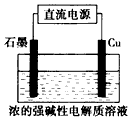
[? ]
A��ͭ�缫������ԭ��Ӧ
B��ʯī�缫�ϲ�������
C��ͭ�缫��ֱ����Դ�ĸ���
D������0.1 mol����ת��ʱ����0.1 mol Cu2O����
�ο��𰸣�B
���������
�����Ѷȣ�һ��
5������� ����ѧ�뼼����ģ��
������һ����ı��أ����Ѻ�ˮ�����ͻ�����������������ȿɽ����ˮ��Դȱ�������⣬�ֿɳ�����ú�����Դ�����Ժ�ˮΪ��Ҫԭ�ϵĺ���ѧ��ҵ���ֱ���Ϊ����ɫ��������
��1�����õĺ�ˮ����������______����______���������������������䶳�������ӽ������ȣ�
��2����ͼ�ǵ�������������ˮ��ԭ��ͼ�����У��缫A��ֱ����Դ���������缫B��ֱ����Դ�ĸ�����
�ٸ�ĤA��______��������ӽ���Ĥ�������ӽ���Ĥ��
�ڴ������۲ɼ��ĺ�ˮ��Ʒ�����������д�����Na+��Cl-���Լ�������K+��SO42-��
��������װ�öԲ��������۵ĺ�ˮ���е�����������������ɺ�A��B��C������������Һ����Һ�壩��pH�ֱ�ΪpHa��pHb��
pHc�������С˳��Ϊ______��
����д���õ��������Բ��������۵ĺ�ˮ���е�������ʱ�������Ļ�ѧ��Ӧ����ʽ______��
��3��������ʱӲ�ȵ�Ӳˮ�ڳ�ʱ�������к����ɳ�������Ҫ�ɷ���______��
��4��Ϊ��ô���ˮ��ȥ����ˮ����ijͬѧ��ʵ���ҽ���Mg2+��Ca2+��Cl-��Ӳˮ�Ⱥ�ͨ�������ӽ�����֬[��RN��CH3��3OH]�������ӽ�����֬[��RSO3H]��д��Cl-���������ӽ�����Ӧ�ķ���ʽ______�����ʵ��δ��óɹ��������ԭ����______��
�ο��𰸣���1����ˮ���������ķ����У�����Ĥ�����䶳�������ӽ������ȣ��ʴ�Ϊ������Ĥ��
��2���������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ������ĤA�����������������������ӷŵ磬���Ը�ĤA�������ӽ���Ĥ���ʴ�Ϊ�������ӽ���Ĥ��
�ڵ��ص������������ӷŵ磬���������������ӷŵ磬��ĤA�������ӽ���Ĥ����ĤC�������ӽ���Ĥ������A�������ԣ�B�������ԣ�C���Լ��ԣ�����pH��С˳��Ϊ��pHa��pHb��pHc���ʴ�Ϊ��pHa��pHb��pHc��
�۵���Ȼ��Ƶķ�Ӧԭ��Ϊ��2NaCl+2H2O?ͨ��?.?2NaOH+H2��+Cl2�����ʴ�Ϊ��2NaCl+2H2O?ͨ��?.?2NaOH+H2��+Cl2����
��3��ˮ������Ҫ�ɷ���������þ��̼��ƣ�Ҳ�Ǿ�����ʱӲ�ȵ�Ӳˮ�ڳ�ʱ�������к����ɳ�������Ҫ�ɷ֣��ʴ�Ϊ��CaCO3��Mg��OH��2��
��4�������ӽ�����֬����ʵ��������֮��Ľ���������Cl-�������ӽ�����֬��Ӧ�ķ���ʽΪ��RN��CH3��3OH+Cl-�TRN��CH3��3Cl+OH-�����Ի����£�þ�������γɳ��������������ӽ��������������ӽ�����֬��ʱ�������ã��ʴ�Ϊ�������ӽ�����֬��������OH-��Mg2+��Ca2+�ȷ�Ӧ���ɳ������������ӽ�������
���������
�����Ѷȣ�һ��