1������� ��֪���з�Ӧ���ʱ�
��1��CH3COOH��l��+2O2��g���T2CO2��g��+2H2O��l����H1=-870.3kJ/mol
��2��C��s��+O2��g���TCO2��g����H2=-393.5kJ/mol
��3��2H2��g��+O2��g���T2H2O��l����H3=-285.8kJ/mol
�Լ��㷴Ӧ?2C��s��+2H2��g��+O2��g���TCH3COOH��l�����ʱ��H=______��
�ο��𰸣���֪����1��CH3COOH��l��+2O2��g���T2CO2��g
���������
�����Ѷȣ�һ��
2��ѡ���� �ڳ��³�ѹ�£���֪��4Fe��s��+3O2��g��=2Fe2O3��s����H1
4Al��s��+3O2��g��=2Al2O3��s����H2
2Al��s��+Fe2O3��s��=Al2O3��s��+2Fe��s����H3
���H3���H1�͡�H2��Ĺ�ϵ��ȷ���ǣ� ? ��
A����H3=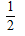 ����H1+��H2��
����H1+��H2��
B����H3=��H2-��H1
C����H3=2����H2+��H1��
D����H3=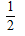 ����H2-��H1��
����H2-��H1��
�ο��𰸣�D
���������
�����Ѷȣ���
3������� ��Դ�Ŀ������������������Ŀɳ�����չϢϢ��أ�����������ú���Դ�ǰ���������ǰ���ش���⣮
I����֪��Fe2O3��s��+3C��ʯī��=2Fe��s��+3CO��g����H=akJ?mol-1
CO��g��+1/2O2��g��=CO2��g����H=bkJ?mol-1
C��ʯī��+O2��g��=CO2��g����H=ckJ?mol-1
��Ӧ��4Fe��s��+3O2��g��=2Fe2O3��s�����ʱ��H=______kJ?mol-1��
��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���______������ţ���
A��C��s��+CO2��g��=2CO��g��
B��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l��
C��2H2O��l��=2H2��g��+O2��g��
D��CH4��g��+2O2��g��=CO2��g��+2H2O��l��
����KOH��ҺΪ�������Һ��������ѡ��Ӧ������Ƴ�һ��ԭ��أ���д����ԭ��صĵ缫��Ӧ��
������______��
������______��
��2���������ȣ�ClO2����һ�ָ�Ч��ȫ������ˮ��������ClO2��һ�ֻ���ɫ���壬������ˮ��ʵ������NH4Cl�����ᡢNaClO2Ϊԭ���Ʊ�ClO2�������£�
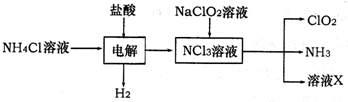
��֪���������з����ķ�ӦΪ��
NH4Cl+2HCl
ͨ��
.
.
NCl3+3H2��������NCl3�е�Ԫ��Ϊ+3�ۣ�
��д�����ʱ�����ĵ缫��Ӧʽ______��
���������Ϸŵ�����ʣ������ӣ���______��
�۳�ȥClO2�е�NH3��ѡ�õ��Լ���______������ţ�
A����ʯ��B����ʯ��C��ŨH2SO4D��ˮ
�������������У�ÿ����1molClO2��������______molNCl3��
�ο��𰸣���Fe2O3��s��+3C��ʯī��=2Fe��s��+3CO��
���������
�����Ѷȣ�һ��
4��ѡ���� ��CH4����ԭNOx�������������������Ⱦ�����磺
��CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)??��H����574?kJ��mol��1
��CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g)???��H����1160?kJ��mol��1
����˵������ȷ���� [???? ]
A.?�ɷ�Ӧ�١��ڿ���֪��CH4(g)��2NO2(g)==N2(g)��CO2(g)��2H2O(g) ��H����867?kJ?mol��1
B. �����ʵ����ļ���ֱ���뷴Ӧ�١��ڣ���Ӧת�Ƶĵ��������
C.?������Ӧ����ʽ�еĻ�ѧ�������ȿ��Ա�ʾ���ʵ������ֿ��Ա�ʾ������
D.?���ñ�״����4.48?L?CH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ1.6NA
�ο��𰸣�C
���������
�����Ѷȣ�һ��
5������� ��֪��
�ٽ�úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)+H2O(g) CO(g)+H2(g)��
CO(g)+H2(g)��
��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C(s) +O2(g)=CO2(g) ��H=-393.5 kJ/mol
CO(g) +l/2O2(g)=CO2(g) ��H= -283.0 kJ/mol
H2(g) +l/2O2(g)=H2O(g) ��H= -242.0 kJ/mol
��ش�
(1)����������Ϣ��д��C(s)��ˮ������Ӧ���Ȼ�ѧ����ʽ��________________________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��� �����ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹����������ͼ��ʾ��ѭ��ͼ�����ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
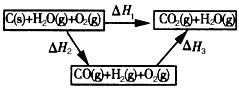
��������ס�����ͬѧ�۵���ȷ����_____���� ���ס����ҡ������жϵ�������_____________����úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ�____________��
�ο��𰸣�(1)C(s) +H2O(g)=CO(g) +H2(g) ��
���������
�����Ѷȣ�һ��
��������ע"91������"���ں�,��30Ԫ,��ȡ����Ա��ҵ���ʦ��������40G