ʱ��:2017-01-17 09:35:09
1��ѡ���� �����£��������ʵ�ˮ��Һ����pHС��7����
A��Na2CO3
B��NH4NO3
C��Na2SO4
D��KNO3
�ο��𰸣�B
�����������ȷ��B
A.????CO32�D��H2O HCO3�D��OH�Dˮ��ʼ���
HCO3�D��OH�Dˮ��ʼ���
B.?????NH4����H2O
�����Ѷȣ���
2��ѡ���� ��֪0��1 mol/L��̼��������Һ��pHΪ8��4��������˵����ȷ���ǣ�????��
A����������NaOH���壬�����Ӻ�̼�������Ũ�Ⱦ�����
B��������Һ��ˮϡ�ͣ�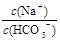 �ı�ֵ���ֲ���
�ı�ֵ���ֲ���
C��c(Na��)��c(H��)��c(HCO3��)��c(CO32��)��c(OH��)
D��c(Na��)��c(HCO3��)��2c(CO32��)��c(H2CO3)
�ο��𰸣�A
���������
���������HCO3-����Һ�д��ڵ���ƽ�⣺ HCO3- H��+CO32����ˮ��ƽ�⣺HCO3-+ H2O
H��+CO32����ˮ��ƽ�⣺HCO3-+ H2O OH
OH
�����Ѷȣ�һ��
3������� A��B������ͬ�����ʣ�����H��N��O��Na�е���������Ԫ����ɵ�ǿ����ʣ�A��ˮ��Һ�ʼ��ԣ�B��ˮ��Һ�����ԣ����ҳ�A��B���ܵ�������ϡ�Ҫ����ͬŨ��ʱ��A1��Һ��ˮ�ĵ���̶�С��A2��Һ��ˮ�ĵ���̶ȣ���ͬŨ��ʱ��B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȡ�����д���пհף�
��1��д����ѧʽ��A1___________��A2__________��B1___________��B2___________��
��2����ͬ�¶��£���A1��B1�����ʵ���Ũ�����ʱ��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ________��
��3��0.1mol?L-1NaNO2��Һ������Ũ���ɴ�С��˳��Ϊ___________________________________��
��4��B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ�ԭ����___________��
��5�������£���B1��B2������Һ��pH��5��������Һ����ˮ������������ӵ����ʵ���Ũ��֮��Ϊ________��
�ο��𰸣���1��NaOH��NaNO2��HNO3��NH4NO3��2��1��
���������
�����������1�����ݸ�����Ԫ�أ�H��N��O��Na������������Ԫ����ɵ�ǿ����ʣ���ѧ��ѧ���������У�ǿ�NaOH����ǿ�ᣨHNO3�����Σ�NaNO3��NaNO2��NH4NO3��NH4NO2�ȡ�A�Լ��ԣ�ֻ������NaOH��NaNO2��B�����ԣ�������HNO3��NH4NO3��ǿ�ᡢǿ�����ˮ�ĵ��룬����ˮ�������ٽ�ˮ�ĵ��롣��ͬŨ��ʱ��A1��Һ��ˮ�ĵ���̶�С��A2��Һ��ˮ�ĵ���̶ȣ���A1ΪNaOH��A2ΪNaNO2����ͬŨ��ʱ��B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ���B1ΪHNO
�����Ѷȣ�һ��
4��ѡ���� �������ӷֱ���봿ˮ�У���ʹˮ��c(OH-)������ǣ���
A��Cl-
B��ClO-
C��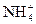
D��Na��
�ο��𰸣�B
���������ClO-��H2O====HClO��OH-��ˮ��ʼ��ԣ�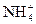 ˮ������ԡ�
ˮ������ԡ�
�����Ѷȣ���
5��ѡ���� �����£����л����Һ�У�������Ũ�ȵĹ�ϵ��ȷ���ǣ�?????��
A��Ũ�Ⱦ�Ϊ0.1mol��L-1��С�մ���Һ���ռ���Һ�������ϣ�
2c��CO32����+c��OH����+c��HCO3������c��H+��=0.1mol��L��1
B��Ũ�Ⱦ�Ϊ0.1mol��L-1�����������Һ������������Һ��������
c��SO42����>c��Na+��>c��NH4+��>c��H+��>c��OH����
C��pH=12�İ�ˮ��pH =2�������������
c��Cl����>c��NH4+��>c��OH����>c��H+��
D��Ũ�Ⱦ�Ϊ0.1mol��L-1�Ĵ�����Һ������������Һ��������
c��Na+��=c��CH3COO����>c��OH����=c��H+��
�ο��𰸣�A
������������⿼��ˮ��Һ������Ũ�ȵıȽ�֪ʶ��Ũ�Ⱦ�Ϊ0.1mol��L-1��С�մ���Һ���ռ���Һ�������Ϻ���Һ���ڵ�����Ϊ��CO32����OH����HCO3����H+��Na+��������Һ���������������ĵ���غ㣬��֪2c��CO32����+c��OH����+c��HCO3����=c��H+��+ c��Na+���������е�c��Na+��=0.1mol��L��1���ɵã�2c��CO32����+c��OH����+c��HCO3������c��H+��=0.1mol��L��1��A�ԣ�Ũ�Ⱦ�Ϊ0.1mol��L-1�����������Һ������������Һ�������Ϻ�õ����ǵ����ʵ���
�����Ѷȣ�һ��