时间:2025-06-25 23:33:40
1、选择题 下列离子方程式书写正确的是(? )
A.Ba(OH)2溶液与过量的NaHCO3溶液混合
Ba2++2OH-+2HCO3- BaCO3↓+CO32-+H2O
BaCO3↓+CO32-+H2O
B.次氯酸钙溶液中通入少量SO2气体:
Ca2++2ClO-+SO2+H2O CaSO3↓+2HClO
CaSO3↓+2HClO
C.金属钾与水反应 2K +2 H2O 2K+ +2 OH-+ H2↑
2K+ +2 OH-+ H2↑
D.醋酸与氢氧化钠溶液的反应 H+ + OH- H2O
H2O
参考答案:C
本题解析:A选项的物料不守恒,应为Ba2++2OH-+2HCO3- BaCO3↓+CO32-+2H2O
BaCO3↓+CO32-+2H2O
B选项则会发生氧化还原反应
D选项的醋酸属于弱电解质,不能拆
本题难度:简单
2、选择题 下列离子方程式正确的是
A.在H2C2O4中加入酸性KMnO4溶液:
B.Ca(HCO3)2与过量Ca(OH)2溶液反应:
C.用惰性电极电解硫酸铜溶液: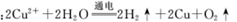
D.足量碳酸氢钠溶液与氢氧化钡溶液混合: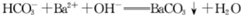
参考答案:A
本题解析:反应符合客观事实,拆写符合电荷守恒、原子守恒。正确。B. 酸式盐与碱发生反应时应该以不足量的物质为标准。由于Ca(OH)2溶液过量 ,所以应该以Ca(HCO3)2为标准。假设其物质的量为1摩尔,反应的方程式为:Ca(HCO3)2+Ca(OH)2 =2CaCO3↓+2H2O。拆写为离子方程式为:Ca2++HCO3-+OH-= CaCO3↓+H2O。反应原理错误。C.用惰性电极电解硫酸铜溶液,由于Cu2+得到电子的能力比H+强,所以Cu2+得到电子;阴离子SO42-、OH-放电能力:OH-> SO42-。所以OH-在阳极放电,产生O2。反应的老祖宗方程式为:2Cu2++ 2H2O 2Cu+ O2↑+4H+.反应原理错误。D. 足量碳酸氢钠溶液与氢氧化钡溶液混合时应该以Ba(OH)2为标准,反应的化学方程式为Ba(OH)2+2NaHCO3=BaCO3↓+2H2O+Na2CO3。反应原理错误。
2Cu+ O2↑+4H+.反应原理错误。D. 足量碳酸氢钠溶液与氢氧化钡溶液混合时应该以Ba(OH)2为标准,反应的化学方程式为Ba(OH)2+2NaHCO3=BaCO3↓+2H2O+Na2CO3。反应原理错误。
本题难度:一般
3、选择题 下列反应的离子方程式书写正确的是
[? ]
A. 氯化铝溶液中加入过量氨水:Al3++4NH3·H2O=AlO2-+4NH4++2H2O
B. 澄清石灰水与少量苏打溶液混合:Ca2++OH-+HCO3-=CaCO3↓+H2O
C. 碳酸钙溶于醋酸:CaCO3+2H+=Ca2++CO2↑+H2O
D. 氯化亚铁溶液中通入氯气:2Fe2++Cl2=2Fe3++2Cl-
参考答案:D
本题解析:
本题难度:一般
4、选择题 下列离子方程式书写正确的是
[? ]
A.钠和水反应:Na+2H2O=Na++2OH-+H2↑
B.二氧化硅与烧碱溶液反应:SiO2 +2OH-= SiO32-+H2O
C.三氯化铁溶液中加入铁粉:Fe3++ Fe= 2Fe2+
D.金属铝溶于氢氧化钠溶液中:2Al+2OH-=2AlO2-+H2↑
参考答案:B
本题解析:
本题难度:一般
5、选择题 下列各组离子,能在强酸溶液中大量共存。并且溶液呈无色的是
A.Mg2+、Na+、SO42-、Cl-
B.K+、Na+、NO3-、MnO4-
C.Na+、Ca2+、OH-、HCO3-
D.NH4+、Cu2+、NO3-、Cl-
参考答案:A
本题解析:离子间如果发生化学反应,则不能大量共存,反之是可以的。A、在无色酸性溶液中Mg2+、Na+、SO42-、Cl-均可以大量共存,A正确;B、MnO4-在酸性溶液中显紫红色,B不正确;C、Ca2+与OH-不能大量共存,OH-与HCO3-也不能大量共存,且在酸性溶液中OH-与HCO3-不能大量共存,C不正确;D、铜离子在溶液中显蓝色,不能大量共存,D不正确,答案选A。
本题难度:一般