ʱ��:2025-06-25 23:08:20
1��ѡ���� ��֪��1u��s��+2H+��aq���T1u2+��aq��+H2��g����H1
2H2O2��l���T2H2O��l��+O2��g����H2
2H2��g��+O2��g���T2H2O��l����H4
��Ӧ1u��s��+H2O2��l��+2H+��aq��=1u2+��aq��+2H2O��l���ġ�H�ǣ� ? ��
A����H=��H1+1/2��H2+1/2��H3
B����H=��H1+1/2��H2-1/2��H3
C����H=��H1+2��H2+2��H3
D����H=2��H1+��H2+��H3
�ο��𰸣�A
���������
�����Ѷȣ���
2��ѡ���� ��298K��100kPaʱ����֪��2H2O(g) �� 2H2(g) + O2(g) ��H1�� Cl2(g) + H2(g) = 2HCl(g) ��H2
2Cl2(g) + 2H2O(g) = 4HCl(g) + O2(g) ��H3�� ��H3�릤H1�ͦ�H2��Ĺ�ϵ��ȷ����
[? ]
A����H3=��H1+2��H2
B����H3=��H1+��H2
C����H3=��H1-2��H2
D����H3=��H1-��H2
�ο��𰸣�A
���������
�����Ѷȣ�һ��
3������� ��Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ��
�� 2H2(g)+O2(g)=2H2O(l) ����H=��570kJ/mol��
�� H2(g)+1/2O2(g)=H2O(g) ����H=-242kJ/mol
�� C(s)+1/2O2(g)=CO(g) ����H= -110��5kJ/moL
�� C(s)+O2(g)=CO2(g) ����H=-393��5kJ/moL
�� CO2(g) +2H2O(g)=2CH4(g) +2O2(g)����H= +890kJ/moL
�ش��������⣺
��1��������Ӧ���������ȷ�Ӧ����____________________________
��2��H2��ȼ����Ϊ_______________��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á���֪C(s) + H2O(g)= H2(g)+ CO (g) ��H=akJ/moL����a=________���÷�Ӧ���ء�S_______0(ѡ���������=����������)��
�ο��𰸣���1����
��2����H=-285kJ/mol
��3��+131.5����
���������
�����Ѷȣ�һ��
4��ѡ���� ����(�Է�ĩ״����)�Ͱ�������������ͬ�������塣��֪��
��Sn(s����)��2HCl(aq)��SnCl2(aq)��H2(g) ��H1
��Sn(s����)��2HCl(aq)��SnCl2(aq)��H2(g) ��H2
��Sn(s����)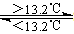 Sn(s����) ��H3����2��1kJ/mol
Sn(s����) ��H3����2��1kJ/mol
����˵����ȷ����
[? ]
�ο��𰸣�D
���������
�����Ѷȣ�һ��
5������� 1840���˹����һϵ��ʵ����ʵ�ó����ɣ���ָ����������һ����Ӧ���Էֲ����У��������Ӧ�ķ�Ӧ���ܺ��������Ӧһ�η���ʱ�ķ�Ӧ����ͬ���������ڸ���Ӧ����ͬ���������ʱ���йط�Ӧ�ȵ���Ҫ���ɣ���Ϊ��˹���ɡ���֪���ʯ��ʯī�ֱ�����������ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C�����ʯ��s)��O2(g)��CO2(g)����H����395��41kJ/mol��
C��ʯī��s����O2(g)��CO2(g)����H����393��51kJ/mol��
����ʯת��ʯīʱ���Ȼ�ѧ����ʽΪ��_____________________���ɴ˿������ȶ���̼��ͬ��������Ϊ��____________����ȡ���ʯ��ʯī��Ͼ��干1mol ��O2����ȫȼ�գ���������ΪQkJ������ʯ��ʯī�����ʵ���֮��Ϊ_____________���ú�Q�Ĵ���ʽ��ʾ����
�ο��𰸣�C�����ʯ��s��=C��ʯī��s������H����1.90kJ/mol��ʯī��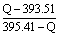
���������
�����Ѷȣ�һ��