时间:2020-08-13 05:39:36
1、选择题 下列反应中盐酸作还原剂的是?(?)
A.CuO +2HCl ="CuCl2" +H2O
B.Fe(OH)3+3HCl =FeCl3 +3H2O
C.Zn+2HCl=ZnCl2 +H2↑
D.2KMnO4 + 16HCl =2KCl+2MnCl2 +5 Cl2↑+ 8H2O
参考答案:D
本题解析:略
本题难度:简单
2、简答题 将一定质量的Na2CO3和NaHCO3混合物灼烧至恒重,将放出的气体通入足量的Ca(OH)2饱和溶液中,充分反应后得到的沉淀的质量是1.0g,加热后剩余的固体物质与足量的稀盐酸反应,收集到2.2g气体,计算原固体混合物中Na2CO3与NaHCO3的质量.(答案保留到小数点后一位).
参考答案:设NaHCO3质量为x,Na2CO3质量为y,依题意得
2NaHCO3?△?.?Na2CO3+H2O+CO2↑
?168?106?44
?x?106x168?44x168
CO2+Ca(OH)2═CaCO3↓+H2O
44? 100
44x168?1.0g
Na2CO3+2HCl═2NaCl+H2O+CO2↑
106?44
y+106x168? 2.2?g
解得x=1.68g,y=4.24g,
答:原固体混合物中Na2CO3的质量为4.24g,NaHCO3的质量为1.68g.
本题解析:
本题难度:一般
3、选择题 下列说法错误的是
A.实验室中少量金属钠通常保存在煤油中。
B.光线通过时,胶体可产生丁达尔效应。
C.氧气、液氯是单质,氯水、氯化氢、纯碱是化合物。
D.“只要工夫深,铁杵磨成针” 只涉及物理变化。
参考答案:C
本题解析:氯气溶于水即得到氯水,所以氯水是混合物,选项C不正确,其余选项都是正确的,答案选C。
点评:该题属于基础题,难度不大。只要能正确把握常见的化学概念,记住常见物质的性质等,就不难得出正确的结论。
本题难度:一般
4、选择题 下列有关化学用语的使用正确的是
A.HCl的电子式: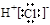
B.镁的原子结构示意图: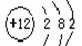
C.溴化钠的电子式: 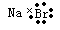
D.Na2SO4的电离方程式:Na2SO4=Na++ SO42-
参考答案:B
本题解析:A、氯化氢是由共价键形成的共价化合物,电子式为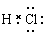 ,A不正确;B、镁是第三正确第ⅡA族元素,其原子结构示意图为
,A不正确;B、镁是第三正确第ⅡA族元素,其原子结构示意图为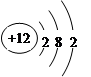 ,B正确;C、溴化钠是含有离子键的离子化合物,电子式为
,B正确;C、溴化钠是含有离子键的离子化合物,电子式为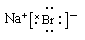 ,C不正确;D、硫酸钠的电离方程式为Na2SO4=2Na++ SO42-,D不正确,答案选B。
,C不正确;D、硫酸钠的电离方程式为Na2SO4=2Na++ SO42-,D不正确,答案选B。
本题难度:简单
5、选择题 下列有关说法正确的是 ( )
A.1 mol Cl2参加反应转移电子数一定为2NA
B.反应Li2NH+H2 LiNH2+LiH中H2既是氧化剂又是还原剂
LiNH2+LiH中H2既是氧化剂又是还原剂
C.在反应KIO3+6HI===KI+3I2+3H2O中,每生成3 mol I2转移的电子数为6NA
D.根据反应中HNO3(稀)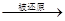 NO,而浓硝酸
NO,而浓硝酸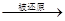 NO2可知,氧化性HNO3(稀)>浓硝酸
NO2可知,氧化性HNO3(稀)>浓硝酸
参考答案:B
本题解析:A、氯气在反应中不一定完全被还原,所以1 mol Cl2参加反应转移电子数不一定为2NA,A错误;B、反应Li2NH+H2 LiNH2+LiH中H2中氢元素的化合价部分升高到+1价,部分降低到-1价,既是氧化剂又是还原剂,B正确;C、在反应KIO3+6HI===KI+3I2+3H2O中,只有5molHI是还原剂,所以每生成3 mol I2转移的电子数为5NA,C错误;D、硝酸的氧化性强弱与还原产物的价态高低无关系,硝酸越浓氧化性越强,D错误,答案选B。
LiNH2+LiH中H2中氢元素的化合价部分升高到+1价,部分降低到-1价,既是氧化剂又是还原剂,B正确;C、在反应KIO3+6HI===KI+3I2+3H2O中,只有5molHI是还原剂,所以每生成3 mol I2转移的电子数为5NA,C错误;D、硝酸的氧化性强弱与还原产物的价态高低无关系,硝酸越浓氧化性越强,D错误,答案选B。
考点:考查阿伏伽德罗常数的计算
本题难度:一般