±Φδ:2017-09-25 16:46:00
1ΓΔ―Γ‘ώΧβ Α―pH=2ΒΡH2SO4ΚΆpH=11ΒΡNaOH»ή“ΚΜλΚΆΘ§ΜλΚΆ“ΚpH=7ΓΘ‘ρΝΫ»ή“ΚΒΡΧεΜΐ±» «Θ®?Θ©
AΘ°10:1
BΘ°1:10
CΘ°1:2
DΘ°2:1
≤ΈΩΦ¥πΑΗΘΚB
±ΨΧβΫβΈωΘΚΜλΚœΚσ»ή“ΚΒΡpHΘΫ7Θ§Υυ“‘«βάκΉ”ΒΡΈο÷ ΒΡΝΩΒ»”ΎOHΘ≠ΒΡΈο÷ ΒΡΝΩΘ§Φ¥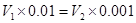 Θ§ΫβΒΟ
Θ§ΫβΒΟ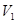 ©U
©U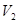 ΘΫ1:10Θ§¥πΑΗ―ΓBΓΘ
ΘΫ1:10Θ§¥πΑΗ―ΓBΓΘ
ΒψΤάΘΚ‘ΎΦΤΥψ»ή“ΚΒΡpH ±Θ§»γΙϊ «ΥαΚΆΦνΖ¥”ΠΘ§‘ρ Ήœ»“Σ≈–ΕœΥαΚΆΦνΒΡΙΐΝΩΈ ΧβΘ§»γΙϊΥαΙΐΝΩΘ§‘ρ Ήœ»ΦΤΥψ»ή“Κ÷–«βάκΉ”≈®Ε»ΓΘ»γΙϊ «ΦνΙΐΝΩΘ§‘ρ Ήœ»ΦΤΥψΒΡ «»ή“Κ÷–OHΘ≠ΒΡ≈®Ε»Θ§»ΜΚσΗυΨίΥ°ΒΡάκΉ”Μΐ≥Θ ΐ‘ΎΜΜΥψ≥…«βάκΉ”≈®Ε»ΓΘ
±ΨΧβΡ―Ε»ΘΚ“ΜΑψ
2ΓΔ―Γ‘ώΧβ (2011ΓΛ»Σ÷ί“Μ÷–ΗΏΕΰΤΎΡ©)œ¬Ν––π ω’ΐ»ΖΒΡ «(ΓΓΓΓ)
AΘ°95 Γφ¥ΩΥ°ΒΡpH<7Θ§ΥΒΟςΦ”»»Ω…ΒΦ÷¬Υ°≥ Υα–‘
BΘ°pHΘΫ3ΒΡ¥ΉΥα»ή“ΚΘ§œΓ Ά10±ΕΚσpHΘΫ4
CΘ°0.2 molΓΛLΘ≠1ΒΡ―ΈΥαΘ§”κΒ»ΧεΜΐΥ°ΜλΚœΚσpHΘΫ1
DΘ°pHΘΫ3ΒΡ¥ΉΥα»ή“ΚΘ§”κpHΘΫ11ΒΡ«β―θΜ·ΡΤ»ή“ΚΒ»ΧεΜΐΜλΚœΚσpHΘΫ7
≤ΈΩΦ¥πΑΗΘΚC
±ΨΧβΫβΈωΘΚ95 Γφ¥ΩΥ°ΒΡpH<7Θ§ΒΪ»ή“Κ≥ ÷––‘Θ§A¥μΈσΘΜ¥ΉΥα‘ΎΦ”Υ°œΓ Ά ±œρΒγάκΒΡΖΫœρ“ΤΕ·Θ§Υυ“‘pH<4Θ§B¥μΈσΘΜ0.2 molΓΛLΘ≠1ΒΡ―ΈΥα”κΒ»ΧεΜΐΥ°ΜλΚœΚσΘ§c(HΘΪ)ΘΫ0.1 molΓΛLΘ≠1Θ§pHΘΫ1Θ§C’ΐ»ΖΘΜpHΘΫ3ΒΡ¥ΉΥα»ή“Κ”κpHΘΫ11ΒΡ«β―θΜ·ΡΤ»ή“Κœύ±»ΫœΘ§¥ΉΥαΒΡ≈®Ε»¥σ”Ύ«β―θΜ·ΡΤ»ή“ΚΒΡ≈®Ε»Θ§Ι Β»ΧεΜΐΜλΚœΚσ¥ΉΥαΙΐΝΩΘ§»ή“Κ≥ Υα–‘Θ§pH<7Θ§D¥μΈσΓΘ
±ΨΧβΡ―Ε»ΘΚ“ΜΑψ
3ΓΔ―Γ‘ώΧβ ≥ΘΈ¬œ¬Θ§œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «
AΘ°0.1 mol/LΒΡNa2SO3»ή“Κ÷–ΝΘΉ”≈®Ε»ΙΊœΒΘΚc(OHΘ≠)ΘΫc(HSO3Θ≠)ΘΪ2c(H2SO3)ΘΪc(H+)
BΘ°ΫΪ≥ΘΈ¬œ¬pHΘΫ12ΒΡ«β―θΜ·ΡΤ»ή“Κ”κpHΘΫ2ΒΡ¥ΉΥα»ή“ΚΒ»ΧεΜΐΜλΚœΚσΘ§»ή“Κ≥ Φν–‘
CΘ°Α―Β»ΧεΜΐΒΡ1ΓΝ10Θ≠5 mol/LΒΡΝρΥα”κ4ΓΝ10Θ≠5 mol/LΒΡNaOH»ή“ΚœύΜλΚœΘ§ΜλΚœ“ΚΒΡpH÷Β”κ5ΓΝ10-6 mol/LΒΡBa(OH)2»ή“ΚΒΡpH÷ΒœύΆ§
DΘ°0.2 molΓΛLΘ≠1CH3COOH»ή“Κ”κ0.1 molΓΛLΘ≠1NaOH»ή“ΚΒ»ΧεΜΐΜλΚœΘΚ2c(HΘΪ)Θ≠2c(OHΘ≠) ΘΫ c(CH3COOΘ≠)Θ≠c(CH3COOH)
≤ΈΩΦ¥πΑΗΘΚB
±ΨΧβΫβΈωΘΚBœνΘΚPHΈΣ2ΒΡ―ΈΥα”κPHΈΣ12ΒΡ«β―θΜ·ΡΤΒ»ΧεΜΐΜλΚœΘ§PHΗ’Η’ΚΟΈΣ7Θ§ΈΣ÷––‘Θ§Ι ¥μΓΘΙ ―ΓBΓΘ
ΒψΤάΘΚ±ΨΧβΩΦ≤ιΘΚΔΌΜλΚœ»ή“Κ÷–άκΉ”≈®Ε»ΒΡΙΊœΒΘ§ΔΎΒ»pHΒΡ»ή“Κ÷–Έο÷ ΒΡΝΩ≈®Ε»ΒΡ¥σ–ΓΙΊœΒΔέΒγάκΚΆΥ°ΫβΒΡœύΜΞΙΊœΒΔήΥα Ϋ―Έ»ή“Κ÷–άκΉ”ΒΡ≈®Ε»¥σ–ΓΙΊœΒΘ§ΉωΧβ ±ΉΔ“βΒγΚ… ΊΚψΓΔΈοΝœ ΊΚψΒΡ‘Υ”ΟΓΘ
±ΨΧβΡ―Ε»ΘΚΦρΒΞ
4ΓΔ―Γ‘ώΧβ œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «ΘΚ
AΘ° Ι”ΟΖ÷ΙβΙβΕ»ΦΤΘ§Ω…“‘ΜΜΥψΒΟΒΫΡ≥»ή“ΚΒΡ≈®Ε»
BΘ°pH ‘÷Ϋ «ΫΪ ‘÷Ϋ”ΟΕύ÷÷ΥαΦν÷Η ΨΦΝΒΡΜλΚœ“ΚΫΰΆΗΘ§Ψ≠ΝάΗ…Κσ÷Τ≥…ΒΡ
CΘ°‘ΎΒΈΕ®Ιΐ≥Χ÷–Θ§ΒΈΦ”»ή“ΚΒΡΥΌΕ»≤ΜΡήΧΪΩλΘ§“ΜΑψ“‘ΟΩΟκ3-4ΒΈΈΣ“Υ
DΘ°“Τ“ΚΙήΙήΦβ≤–ΝτΒΡ“ΚΧε“ΜΕ®“Σ”Οœ¥Εζ«ρ¥Β≥ω“‘±Θ÷Λ“Τ»Γ“ΚΧεΧεΜΐΒΡΉΦ»Ζ–‘
≤ΈΩΦ¥πΑΗΘΚD
±ΨΧβΫβΈωΘΚΩΦ≤ιΜυ±ΨΒΡ Β―ι≤ΌΉςΓΘ“Τ“ΚΙή «”Οά¥ΉΦ»Ζ“Τ»Γ“ΜΕ®ΧεΜΐΒΡ»ή“ΚΒΡΝΩΤςΘ§“Τ“ΚΙή «“Μ÷÷ΝΩ≥ω Ϋ“«ΤςΘ§÷Μ”Οά¥≤βΝΩΥϋΥυΖ≈≥ω»ή“ΚΒΡΧεΜΐΓΘΥυ“‘“Τ“ΚΙήΙήΦβ≤–ΝτΒΡ“ΚΧε≤ΜΡή”Οœ¥Εζ«ρ¥Β≥ωΘ§Ζώ‘ρΝΩ»ΓΒΡΧεΜΐΨΆΤΪ¥σΓΘ
±ΨΧβΡ―Ε»ΘΚ“ΜΑψ
5ΓΔ―Γ‘ώΧβ ΙΊ”Ύ≥ΘΈ¬œ¬pHΘΫ3ΒΡ¥ΉΥα»ή“ΚΘ§œ¬Ν––π ω’ΐ»ΖΒΡ «
AΘ°Φ”Υ°œΓ Ά10±ΕΚσΘ§pHΘΫ4
BΘ°Φ”Υ°œΓ ΆΚσΘ§»ή“Κ÷–c(H+)ΚΆc(OHΘ≠)ΨυΦθ…Ό
CΘ°Φ”»κ¥ΉΥαΡΤΨßΧεΚσΘ§»ή“ΚΒΡpH‘ω¥σ
DΘ°Φ”»κΒ»ΧεΜΐΓΔpHΘΫ11ΒΡNaOH»ή“ΚΚσΘ§c(Na+)ΘΫ c(CH3COOΘ≠)
≤ΈΩΦ¥πΑΗΘΚC
±ΨΧβΫβΈωΘΚ¥ΉΥα «»θΒγΫβ÷ Θ§¥φ‘ΎΒγάκΤΫΚβΓΘ
AΘ°Φ”Υ°œΓ ΆΜα¥ΌΫχ¥ΉΥαΒΡΒγάκΘ§Ι œΓ Ά10±ΕΚσΘ§pH<4ΓΘA¥μΈσΓΘ
BΘ°Φ”Υ°œΓ ΆΚσΘ§»ή“Κ÷–c(H+)Φθ–ΓΘ§ΒΪΥ°ΒΡάκΉ”Μΐ≥Θ ΐ≤Μ±δΘ§Ι c(OHΘ≠)‘ω¥σΘ§B¥μΈσΓΘ
CΘ°Φ”»κ¥ΉΥαΡΤΨßΧεΘ§¥ΉΥαΗυάκΉ”≈®Ε»‘ω¥σΘ§Μα“÷÷Τ¥ΉΥαΒΡΒγάκΘ§«βάκΉ”≈®Ε»Φθ–ΓΘ§pH‘ω¥σΘ§C’ΐ»ΖΓΘ
DΘ°Φ”»κΒ»ΧεΜΐΓΔpHΘΫ11ΒΡNaOH»ή“ΚΚσΘ§¥ΉΥαΜΙ”–¥σΝΩΒΡ Θ”ύΘ§»ή“Κœ‘Υα–‘Θ§c(Na+)< c(CH3COOΘ≠)Θ§D¥μΈσΓΘ
Ι ―ΓC
±ΨΧβΡ―Ε»ΘΚ“ΜΑψ