时间:2017-08-08 06:45:14
1、选择题 金刚石和石墨都是碳的单质,石墨在一定条件下可以转化为金刚石并需要吸收能量。已知12g石墨或金刚石完全燃烧时放出的热量依次为Q1和Q2,下列说法错误的是
[? ]
A.Q1< Q2
B.石墨不如金刚石稳定
C.石墨具有的能量比金刚石低
D.石墨与金刚石完全燃烧,生成的二氧化碳一样多
参考答案:B
本题解析:
本题难度:一般
2、选择题 已知:
Fe2O3(s)+3/2C(s)=3/2CO2(g)+2Fe(s) △H=234.1 kJ/mol
C(s)+O2(g)=CO2(g) △H= -393. 5 kJ/mol
则2Fe(s)+3/2O2(g)=Fe2O3(s)的△H值是
[? ]
A.-824.4 kJ/mol
B.-627.6 kJ/mol
C.-744.7 kJ/mol
D.-169.4 kJ/mol
参考答案:A
本题解析:
本题难度:一般
3、简答题 研究化学反应对人类社会的发展进步有重要意义.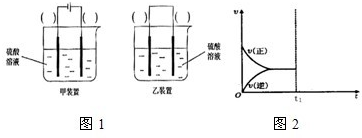
(1)已知:N2(g)+3H2(g)2NH3(g)△H1=-92.4kJ/mol
2H2(g)+O2(g)=2H2O(g)△H2=-523.6kJ/mol
H2O(g)=H2O(l)△H3=-44.0kJ/mol
根据最新“人工固氮”的研究报道,在常温、常压、光照条件下,N2在催化剂(掺有少量Fe2O3的TiO2)表面与水发生反应生成氨气,试写出此反应的热化学方程式______.
(2)现有反应:Cu+H2SO4═CuSO4+H2,欲通过如图1所示装置实现上述反应,请选择合适的装置在括
号内标出电极材料(填“Cu”或“C”),并且写出C电极上的电极反应式______.
(3)长期使用的锅炉需要定期除水垢,否则会降低燃料的利用率.水垢中含有的CaSO4,可先用Na2CO3溶液处理,使之转化为疏松、易溶于酸的CaCO3,而后用酸除去.则CaSO4?转化为CaCO3的离子方程式为______.
(4)科学研究发现纳米级Cu2O的可作为太阳光分解水的催化剂.一定温度下,在2L密闭容器中加入纳米级Cu2O并通入0.10mol水蒸气,发生反应:2H2O(g)
| 时间/min | 20 | 40 | 60 | 80 n(O2)/mol 0.0010 0.0016 0.0020 0.0020 |
参考答案:解(1)已知:①N2(g)+3H2(g)2NH3(g)△H1=-92.4kJ/mol
②2H2(g)+O2(g)=2H2O(g)△H2=-523.6kJ/mol
③H2O(g)=H2O(l)△H3=-44.0kJ/mol
则反应2N2(g)+6H2O(l)=4NH3(g)+3O2(g)等于①×2-3×②-6×③,所以△H=2△H1-3△H2-6△H3=-1650?kJ/mol,
故答案为:2N2(g)+6H2O(l)=4NH3(g)+3O2(g)△H=-1650?kJ/mol;
(2)Cu和H2SO4之间的反应是非自发的,需要电解池实现,金属铜作阳极,阴极是导体即可,电解质为硫酸,即: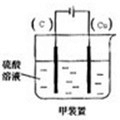
,
C电极为阴极,反应为:2H++2e-→H2↑,故答案为:
;2H++2e-→H2↑;
(3)沉淀向着更难溶的方向转化,CaSO4和碳酸钠反应转化为CaCO3的离子方程式为:CaSO4(s)+CO32-(aq)=CaCO3(s)+SO42-(aq),
故答案为:CaSO4(s)+CO32-(aq)=CaCO3(s)+SO42-(aq);
(4)①根据表中数据,可以看出反应在60min时达到平衡,则
? 2H2O(g)光
本题解析:
本题难度:一般
4、选择题 已知H2(g)、C2H4(g)和C2H5OH(1)的燃烧热分别是-285.8kJ·mol-1、-1411.0kJ·mol-1和
-1366.8kJ mol-1,则由C2H4(g)和H2O(l)反应生成C2H5OH(l)的△H为
[? ]
参考答案:D
本题解析:
本题难度:一般
5、填空题 广州亚运“潮流”火炬燃料是丙烯(C3H6),北京奥运会“祥云”火炬燃料是丙烷(C3H8)(1)丙烷脱氢可得丙烯.
已知:C3H8(g)═CH3CH=CH2?(g)+H2(g)△H1=124.2kJ?mol-1
CH3CH=CH2(g)═CH4(g)+HC≡CH(g)△H2=32.4kJ?mol-1
(1)则相同条件下,反应C3H8(g)═CH4(g)+HC≡CH(g)+H2(g)的△H=______kJ?mol-1.
(2)以丙烷为燃料制作新型燃料电池,电池的正极通入O2和CO2,负极通入丙烷,电解质是熔融碳酸盐.电池反应方程式为______;放电时,CO32-移向电池的______(填“正”或“负”)极.
参考答案:(1)已知:①、C3H8(g)═CH3CH=CH2?(g)+H2(g)△H1=124.2kJ?mol-1
②、CH3CH=CH2(g)═CH4(g)+HC≡CH(g)△H2=32.4kJ?mol-1
根据盖斯定律,①+②得C3H8(g)═CH4(g)+HC≡CH(g)+H2(g),故△H=124.2kJ?mol-1+32.4kJ?mol-1=156.6kJ?mol-1,
故答案为:156.6;
(2)负极通入丙烷,碳元素的化合价升高,电池的正极通入O2,氧元素的化合价降低,即丙烷与氧气反应生成二氧化碳和水,则电池的总反应为C3H8+5O2═3CO2+4H2O,原电池中阴离子向负极移动,即CO32-移向电池的负极,
故答案为:C3H8+5O2═3CO2+4H2O;负.
本题解析:
本题难度:一般