时间:2017-08-06 10:03:17
1、选择题 北京大学和中国科学院的化学工作者已成功研制出碱金属与C60形成的球碳盐K3C60,实验测知该物质属于离子化合物,具有良好的超导性。下列有关分析正确的是(?)
A.K3C60中只有离子键
B.K3C60中不含共价键
C.该晶体在熔融状态下能导电
D.C60与12C互为同素异形体
2、选择题 已知(OCN)2的电子式为: ,则SCN-的电子式正确的是(?)
,则SCN-的电子式正确的是(?)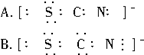

3、选择题 同一周期的两主族元素A、B能形成AB2型离子化合物,A、B元素在周期表中的族序数可能为( )
A.ⅡA、ⅦA
B.IA、ⅦA
C.ⅣA、ⅥA
D.IA、VA
4、填空题 L、M、Q、R、X代表五种物质,它们都含某种价态的氮元素,各物质中氮元素的化合价只有一种.物质L中氮元素的化合价比物质M中氮元素的化合价低.在一定条件下,它们会有如下的转化关系(未配平).
Q+HCl→M+Cl2+H2O
R+L→X+H2O
R+O2→L+H2O
请判断:
(1)五种物质按氮元素的化合价从高到低的顺序排列是______,
若五种物质中有一种是硝酸,那么硝酸应该是______(用字母表示)
(2)某同学写出下面不同价态的氮的化合物相互转化关系(未配平),其中你认为一定不能实现的是______
A.NO+HNO3→N2O3+H2OB.NH3+NO→HNO2+H2OC.N2O4+H2O→HNO3+HNO2.
5、选择题 下列物质中,既含有离子键,又含有共价键的是( )
A.HF
B.Ca(OH)2
C.CaO
D.SO2