时间:2017-07-27 10:22:58
1、选择题 下列药品置于空气中很容易变质(括号内的物质是变质后的物质),其中不是因为氧化还原反应而变质的是(? )
A.Na2SO3(Na2SO4)
B.FeCl2(FeCl3)
C.KI(I2)
D.NaOH(Na2CO3)
2、选择题 下列有关氢化物的叙述中正确的是( )
A.在卤化氢中HF最稳定
B.一个D2O分子所含的中子数为8
C.稳定性:H2S>HF
D.HCl的电子式为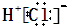
3、选择题 下列说法不正确的是
A.碱土金属都可用于制造焰火
B.白磷和红磷常温下都是固体,且都能被空气氧化
C.过渡元素都是金属元素,且都是电的良导体
D.镧系15种元素的化学性质十分相似
4、选择题 元素性质呈周期性变化的决定因素是
A.元素的原子半径大小呈周期性变化
B.元素相对原子质量依次递增
C.元素原子的核外电子排布呈周期性变化
D.元素的主要化合价呈周期性变化
5、选择题 下列排列顺序正确的是
[? ]
①热稳定性:H2O>HF>H2S
②原子半径:Na>Mg>O
③酸性:H3PO4>H2SO4>HClO4
④结合质子能力:OH->CH3COO->Cl-
A.①③
B.②④
C.②③
D.①④