ʱ��:2017-07-18 01:11:26
1��ѡ���� ����Ԫ�ص�ԭ�����γɲ�ͬ����ʱ�������γ����Ӽ��������γɹ��ۼ����ǣ�������
A��K
B��Ca
C��I
D��Ne
2��ѡ���� �Ӻ�ˮ����ȡ�������·�Ӧ��5NaBr+NaBrO3+3H2SO4�T3Br2+3Na2SO4+3H2O����÷�Ӧ��������ԭ��Ӧԭ���������Ƶ���
A.2NaBr+Cl2�T2NaCl+Br2
B.C+CO22CO
C.2H2S+SO2�T2H2O+3S��
D.AlCl3+3NaAlO2+6H2O�T4Al��OH��3��+3NaCl
3�������� ��֪��101 kPaʱ��CO��ȼ����Ϊ283 kJ��mol-1����ͬ�����£���2 mol CH4��ȫȼ������Һ̬ˮ�����ų�������Ϊ1 mol CO��ȫȼ�շų�������6.30���������ȼ�����Ƕ��٣�
��2������CO��CH4��ɵĻ�����干0.75 mol����ȫȼ�����ɶ�����̼�����18 gҺ̬ˮ���ų�����Ϊ?���٣�?
��3��120 �桢101 kPa�£�a mL��CO��CH4��ɵĻ��������b mL O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ���������������O2ǡ����ȫ��Ӧ������b mL CO2������������CH4���������Ϊ____________������2λС������
����ȼ�պ����������С��a/4 mL����a��b��ϵ����ѧ��ʾʽ��__________________��
4��ѡ���� ��������������ǣ�������
A�����෴�������֮����������Ϊ���Ӽ�
B������Ԫ����ǽ���Ԫ�ػ���ʱ��һ���γ����Ӽ�
C��ijԪ�ص�ԭ�������ֻ��һ�����ӣ�����±�ؽ��ʱ���γɵĻ�ѧ����һ�������Ӽ�
D��ϡ�������в����ڻ�ѧ��
5��ѡ���� ����˵�����ʾ��������ȷ����
A�����������������������ֱ���ȫȼ�գ����߷ų���������
B����C��ʯī�� C�����ʯ�� ��H��+19 kJ��mol-1��֪�����ʯ��ʯī�ȶ�
C�����ʯ�� ��H��+19 kJ��mol-1��֪�����ʯ��ʯī�ȶ�
C����101 kPaʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ����������ȼ�յ��Ȼ�ѧ����ʽΪ��H2��g��+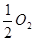 (g)====H2O(l) ��H��+285.8 kJ��mol-1
(g)====H2O(l) ��H��+285.8 kJ��mol-1
D����ϡ��Һ�У�H+��aq��+OH-(aq)====H2O(l) ��H����57.3 kJ��mol-1������0.5 molŨ������Һ�뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ