1
2
O2��g���TCO2��g����H=-283.0kJ?mol-1
��ش�
��1�������������ݣ�д��C��s����ˮ������Ӧ���Ȼ�ѧ��Ӧ����ʽ��______��
��2���ȽϷ�Ӧ�����ݿ�֪��1molCO��g����1molH2��g����ȫȼ�շų�������֮�ͱ�1molC��s����ȫȼ�շų��������࣮��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹������������ѭ��ͼ����ͼ����
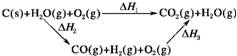
���ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
��������ס�����ͬѧ�۵���ȷ����______����ס����ҡ������жϵ�������______��
��3����úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ�______��
______��
��4��ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϣ�
CO��H2��һ�������¿��Ժϳɣ��ټ״����ڼ�ȩ���ۼ��ᡢ�����ᣮ
�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����______������ţ�����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷţ�
�ο��𰸣���1����C��ʯī��s��+O2��g���TCO2��g����H=-39
���������
�����Ѷȣ�һ��
2��ѡ���� ��֪��2Zn��s��+O2��g��=2ZnO��s������H=-701.0kJ?mol-1
2Hg��l��+O2��g��=2HgO��s������H=-181.6kJ?mol-1
��ӦZn��s��+HgO��s��=ZnO��s��+Hg��l���ġ�HΪ�� ? �� A��+519.4kJ?mol-1
B��+259.7?kJ?mol-1
C��-259.7kJ?mol-1
D��-519.4kJ?mol-1
�ο��𰸣�C
���������
�����Ѷȣ���
3������� ��Դ��ȱ������������ٵ��ش����⡣�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦI�� CO(g)��2H2(g)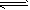 CH3OH(g) ��H1 CH3OH(g) ��H1
��ӦII��CO2(g)��3H2(g)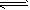 CH3OH(g) + H2O(g) ��H2 CH3OH(g) + H2O(g) ��H2
���±����������Ƿ�ӦI�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
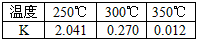
�ɱ��������жϦ�H1______0 �����������������������
��ij�¶��£���2 mol CO��6 mol H2����2L���ܱ������У�5min��÷�Ӧ�ﵽƽ�⣬���c(CO)��0.2
mol/L������H2��ʾ�ĸ÷�Ӧ�ķ�Ӧ����Ϊ��________����ʱ���¶�Ϊ________�����ϱ���ѡ��
��2����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) ��2CO2(g) �� 4H2O(g) ��H ����1275.6 kJ/mol
�� 2CO (g)+ O2(g) ��2CO2(g) ��H ����566.0 kJ/mol
�� H2O(g) ��H2O(l) ��H ����44.0 kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��______________________
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�á�
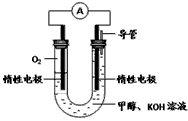
�ٸõ�������ĵ缫��ӦΪ__________________��
���øõ�ص��200mL����ʳ��ˮ(��������)��һ��ʱ��������ñ��������2.24L��������Һ��pHΪ________����Һ����仯���Բ��ƣ���Ҫʹ������Һ��ԭ����������Ϊ___________�������ƣ���
�ο��𰸣���1���٣�����0.32mol��L-1��min-1��250��
���������
�����Ѷȣ�һ��
4��ѡ���� ��֪298Kʱ������Ӧ��
��C��s��+1/2O2��g��=CO��g����H1=-110.5kJ?mol-1
��CO��g��+1/2O2��g��=CO2��g����H2=-283.0kJ?mol-1
��C��s��+CO2��g��=2CO��g����H3��
���H3���ڣ� ? �� A��172.5kJ?mol-1
B��-172.5kJ?mol-1
C��393.5kJ?mol-1
D��-393.5kJ?mol-1
�ο��𰸣�A
���������
�����Ѷȣ�һ��
5������� ���³�ѹ�£�����1mol�����룩������ӻ�ѧ�������յ��������γ�1mol�����룩������ӻ�ѧ�����ų���������Ϊ���ܣ���λΪkJ/mol���±���һЩ�������ݣ�kJ��mol-1��
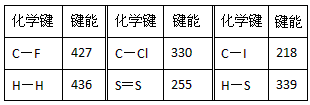
�ش��������⣺
��1���ɱ������ݹ���Ԥ��C��Br���ļ��ܷ�Χ��_________<C��Br����<_________
��2���Ȼ�ѧ����ʽ2H2(g)+S2(g) ==2H2S(g) ��H= QkJ��mol-1����Q=___________
��3����֪�����Ȼ�ѧ����ʽ��
O2(g) == O2+(g) +e- ��H1= +1175��7 kJ��mol-1
PtF6(g) + e-== PtF6-(g) ��H2= -771��1 kJ��mol-1
O2+PtF6-(s) == O2+(g) + PtF6-(g) ��H3= +482��2 kJ��mol-1
��ӦO2(g) +_________(g) = O2+PtF6-(s)�ġ�H=_____________ kJ��mol-1��
�ο��𰸣���1��218kJ��mol-1��330kJ��mol-1
���������
�����Ѷȣ�һ��
��������ע"91������"���ں�,��30Ԫ,��ȡ����Ա��ҵ���ʦ��������40G
|