1��ѡ���� ��֪0.1mol/L��ʽ��NaHA����ҺpH=5��������˵����ȷ���ǣ�������
A��NaHA�ĵ��뷽��ʽΪNaHA�TNa++H++A-
B��HA-��ˮ��������HA-�ĵ���
C��c��A2-����c��H2A��
D��c��OH-��=c��HA-��+c��A2-��+c��H2A��
�ο��𰸣�C
���������
�����Ѷȣ�һ��
2��ѡ���� ���������� �� NaCl��Һ �� ���ڵ�MgCl2 �� CuSO4��ĩ �� NaOH ���� �� ����ͭ �� ���ǡ��й����ǵ�˵����ȷ����[???? ]
A. �ܵ�����Т٢ڢۢܢ�
B. ���ڵ�����Т٢ڢۢ�
C. ���ܵ�����Тۢܢ�
D. ���ڴ������ֻ�Тܢݢ�
�ο��𰸣�C
���������
�����Ѷȣ���
3��ѡ���� ��һ�������Ba��OH��2��Һ����μ���ϡ���ᣬ��Һ�ĵ����ԣ��Ե���I��ʾ�������ϡ������������V��ʾ����Ĺ�ϵ��ӽ���ʵ������ǣ� ? ��
A��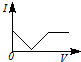
B��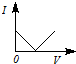
C��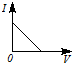
D��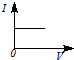
�ο��𰸣�A
���������
�����Ѷȣ�һ��
4��ѡ���� ij��Һ�к���NH4+��H+��SO42-��OH-���Ը���Һ������һ����ȷ���ǣ� ? ��
A������Һ���ܳ����ԣ�Ҳ���ܳʼ��Ի�����
B��ֻ��������狀�����Ļ����Һ
C������Ũ���ɴ�С��˳���ǣ�c��NH4+����c��SO42-����c��H+����c��OH-��
D������Ũ�ȴ������й�ϵ��c��NH4+��+c��H+��=c��OH-��+c��SO42-��
�ο��𰸣�A
���������
�����Ѷȣ�һ��
5��ѡ���� ����˵����ȷ����[???? ]
A���������ǵ���ʵı�����������ˮ��Һ���ۻ�״̬���ܷ�
B��ǿ�������������ʵı�����������ˮ��Һ�����Ե�ǿ��
C���ᡢ���������ڵ���ʣ����������ﶼ�Ƿǵ����
D��������ǿ�ᡢǿ��ʹ��ζ���ǿ����ʣ����������ﶼ�Ƿǵ����
�ο��𰸣�A
���������
�����Ѷȣ���
��������ע"91������"���ں�,��30Ԫ,��ȡ����Ա��ҵ���ʦ��������40G