ʱ��:2017-01-13 10:26:10
1��ѡ���� K35ClO3��K37Cl��������Һ�з�Ӧ���������������������Է�������Ϊ
A.70.7
B.70.2
C.72
D.73.3
�ο��𰸣�D
�������������������ԭ��Ӧ�е��ӵĵ�ʧ�غ��֪�������ɵ�������35Cl��37Cl��ԭ�Ӹ���֮����1�U5��������������ԭ�ӵ����ԭ��������35��1/6��37��5/6=36.67,������������Է���������36.67��2��73.3����ѡD��
�����Ѷȣ���
2��ѡ���� ������һ����̼�����顢����ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
H2(g)+O2(g)=H2O(l)����H=��285.8kJ/mol
CO(g)+ 1/2O2(g)=CO2(g)����H=��282.6kJ/mol
C8H18(l)+O2(g)=8CO2(g)+9H2O(l)����H=��5472kJ/mol
CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H=��889.6kJ/mol
��ͬ������������һ����̼�����顢������ȫȼ��ʱ���ų��������ٵ��� [???? ]
A��H2(g)
B��CO(g)
C��C8H18(l)
D��CH4(g)
�ο��𰸣�B
���������
�����Ѷȣ�һ��
3��ѡ���� ʵ������4 mol SO2��2 mol O2�������з�Ӧ��2SO2(g)+O2(g) 2SO3(g) ��H= -196. 64 kJ/mol�����ų�
2SO3(g) ��H= -196. 64 kJ/mol�����ų�
314. 624 kJ����ʱ��SO2��ת����Ϊ
[???? ]
�ο��𰸣�C
���������
�����Ѷȣ���
4������� ����ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
��1�����ڸ÷�Ӧ������˵���У���ȷ����______��
A����H��0����S��0��B����H��0����S��0��
C����H��0����S��0��D����H��0����S��0��
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ______��
��3���¶Ƚ��ͣ�ƽ�ⳣ��K______������������䡱��С������
��4��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L���ܱ������У�����1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v��H2��______mol?L-1?min-1��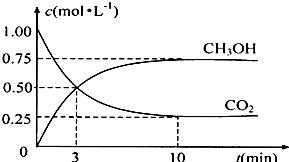
��5�����д�ʩ����ʹ
| n(CH3OH) n(CO2) �������______�� A�������¶ȣ�B����������� C����H2O��g������ϵ�з��룻D������He��g����ʹ��ϵ��ѹǿ���� 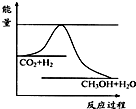 �ο��𰸣���1����ͼ���֪����Ӧ��������ߣ�������������ͣ��÷���Ϊ�� ��������� �����Ѷȣ��� 5�������� ���ӹ�ҵ��������Ƭ�ϵĶ�������ķ�Ӧ�ǣ�SiO2(s)+4HF(g)==SiF4(g)+2H2O(g) ���H��298K��== -94.0kJ��mol-1 ��S(298K)== -75.8J��mol-1��K-1 ���H�͡�S�����¶ȱ仯���仯������˷�Ӧ�Է����е��¶������� �ο��𰸣�T��1.24��103K ��������� �����Ѷȣ�һ�� |