时间:2016-12-26 20:51:01
选择题 已知:O2(g) = O2+(g)+e- 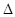 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e- 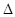 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S) 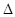 H3=" -482.2"来源:91 考试网 kJ·mol-1
H3=" -482.2"来源:91 考试网 kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6 (s)的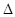 H是( )
H是( )
A.77.6 kJ
B.-77.6 kJ·mol-1
C.+77.6kJ·mol-1
D.-886.8kJ·mol-1
选择题 已知:O2(g) = O2+(g)+e- 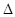 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e- 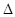 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S) 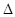 H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6 (s)的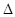 H是( )
H是( )
A.77.6 kJ
B.-77.6 kJ·mol-1
C.+77.6kJ·mol-1
D.-886.8kJ·mol-1
本题答案:B
本题解析:运用盖斯定律,将O2(g) = O2+(g)+e-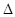 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e-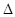 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S) 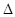 H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1
三个热化学反应方程式叠加,可得:O2(g)+PtF6(g) = O2PtF6 (s)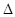 H= -77.6 kJ·mol-1
H= -77.6 kJ·mol-1
本题所属考点:【热化学方程式】
本题难易程度:【简单】