ʱ��:2020-08-13 07:22:27
1������� ��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��úֱ��ȼ��C��s��+O2?��g���TCO2��g����H1��0?��
;��II�����Ƴ�ˮú����C��s��+H2O��g���TCO��g��+H2��g����H2��0?��
��ȼ��ˮú����2CO��g��+O2��g���T2CO2��g����H3��0?��
2H2��g��+O2?��g���T2H2O��g����H4��0?��
��ش��������⣺
��1��ȼ�յ�����ú��;��I�ų�������______������ڡ������ڡ���С�ڡ���;��II�ų���������������������______��
��2����H1����H2����H3����H4����ѧ��ϵʽ��______��
2��ѡ���� ��298K��100kPaʱ����֪��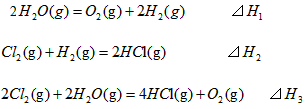
��SH1��SH2�ͨSH3��Ĺ�ϵ��ȷ����
[? ]
A��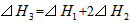
B��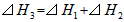
C��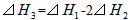
D��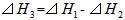
3��ѡ���� ��֪��ѧ��Ӧ����ЧӦֻ�뷴Ӧ��ij�ʼ״̬�������������״̬�йأ���ͼ������ʾ����H1=��H2+��H3����������ԭ����ͼ������ʾ���жϸ���Ӧ�ķ�Ӧ�ȹ�ϵ�в���ȷ���ǣ� ? ��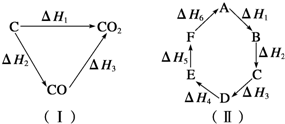
A��A��F��H=-��H6
B��A��D��H=��H1+��H2+��H3
C����H1+��H2+��H3+��H4+��H5+��H6=0
D����H1+��H6=��H2+��H3+��H4+��H5
4��ѡ���� ��֪
��l�� ��H2O��g�� ��H1��a kJ��
��H2O��g�� ��H1��a kJ��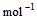
��2��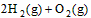 ��2H2O��g�� ��H2��b kJ��
��2H2O��g�� ��H2��b kJ��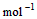
��3��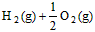 ��H2O��l�� ��H3��c kJ��
��H2O��l�� ��H3��c kJ��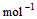
��4�� ��2H2O��l�� ��H4��d kJ��
��2H2O��l�� ��H4��d kJ�� 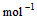
���й�ϵʽ����ȷ����
[? ]
A��a��c ��0
B��b��d��0
C��2a��b��0
D��2c��d��0
5������� ��1����֪���з�Ӧ��
2CO��g��+SO2��g���TS��g��+2CO2��g����H=+8.0kJ?mol-1����
2H2��g��+SO2��g���TS��g��+2H2O��g����H=+90.4kJ?mol-1��
2CO��g��+O2��g���T2CO2��g����H=-566.0kJ?mol-1��
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1��
��S��g����O2��g����Ӧ����SO2��g�����Ȼ�ѧ����ʽ�ɱ�ʾΪ______
��2��NH4Cl��Һ��______�ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��______
��3�������£�pH=11��CH3COONa��Һ�У�ˮ���������c��OH-��=______��
��4����֪ˮ��������ƽ�⣺H2O+H2O?H3O++OH-��H��0������ʹƽ�������ƶ�����������Һ�����ԣ�ѡ����______��
A����ˮ�м���NaHSO4����B����ˮ�м�Na2CO3����
C��������100��[����c��H+��=1��10-6mol?L-1]
D����ˮ�м��루NH4��2SO4����
��5������Ũ�ȡ��������NaOH��Һ��NH3?H2O��Һ�ֱ��ˮϡ��m����n����ϡ�ͺ�������Һ��pH��ȣ���m______n�����������������=������