时间:2020-07-28 04:08:04
1、选择题 在含有NaBr、KI的混合溶液里通入足量的Cl2,充分反应后将溶液蒸干,然后灼烧残余固体,最后剩下的是
A.氯化钾和碘
B.氯化钠和溴
C.氯化钾、氯化钠和碘
D.氯化钾和氯化钠
2、填空题 已知有机物 A、B、C、D、E、F之间的转化关系如图所示。D 是常用做汽车发动机的抗冻剂,E为聚合物,F的式量为26。根据信息回答下列问题。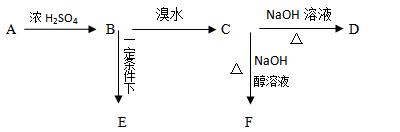
(1)?写出A中官能团名称??
(2)? B转化为C的反应类型?
(3)?有机物D具有以下哪几方面的化学性质:?(填序号)
①?加成反应?②取代反应?③消去反应?④氧化反应?⑤?还原反应
(4)? E的结构简式为?
(5)? C—F反应的方程式?
3、选择题 下列各组液体混合物,用分液漏斗不能分离的是(? )
A.溴乙烷和水
B.氯乙烷和稀盐酸
C.甲苯和水
D.苯和溴苯
4、选择题 下列不属于消去反应的是(?)
5、选择题 C5H11Cl发生消去反应,得到两种烯烃,则该卤代烃的结构简式可能为(?)
A.CH3(CH2)4Cl
B.CH3CH2CHClCH2CH3
C.CH3CHCl(CH2)2CH3
D.(CH3)3CCH2Cl