时间:2019-07-04 01:26:39
1、选择题 已知:
(1)Zn(s)+1/2O2(g)===ZnO(s) ΔH=-348.3?kJ/mol
(2)2Ag(s)+1/2O2(g)===Ag2O(s) ΔH=-31.0?kg/mol
则Zn与Ag2O?反应生成ZnO和Ag的热化学方程式为
[?]
A.Zn(s)+Ag2O(s)===ZnO(s)+2Ag(s) ΔH=317.3?kJ/mol
B.Zn+Ag2O===ZnO+2Ag ΔH=317.3?kJ/mol
C.Zn(s)+Ag2O(s)===ZnO(s)+2Ag(s) ΔH=-317.3?kJ/mol
D.2Zn(s)+2Ag2O(s)===2ZnO(s)+4Ag(s) ΔH=-317.3?kJ/mol
2、选择题 已知下列数据:
Fe(s)+1/2O2(g) =FeO(s) △H=-272kJ·mol-1
2Al(s)+3/2O2(g) =Al2O3(s) △H=-1675kJ·mol-1
则2Al(s) +3FeO(s) =Al2O3(s) +3Fe(s)的△H是
[? ]
A.+859 kJ·mol-1
B.-859 kJ·mol-1
C.-1403 kJ·mol-1
D.-2491 kJ·mol-1
3、选择题 已知:1u(s)+2H+(aq)═1u2+(aq)+H2(g)△H1
2H2O2(l)═2H2O(l)+O2(g)△H2
2H2(g)+O2(g)═2H2O(l)△H4
则反应1u(s)+H2O2(l)+2H+(aq)=1u2+(aq)+2H2O(l)的△H是( ? )
A.△H=△H1+1/2△H2+1/2△H3
B.△H=△H1+1/2△H2-1/2△H3
C.△H=△H1+2△H2+2△H3
D.△H=2△H1+△H2+△H3
4、选择题 灰锡(以粉末状存在)和白锡是锡的两种同素异形体。已知:
①Sn(s、白)+2HCl(aq)=SnCl2(aq)+H2(g) △H1
②Sn(s、灰)+2HCl(aq)=SnCl2(aq)+H2(g) △H2
③Sn(s、灰)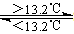 Sn(s、白) △H3=+2.1kJ/mol
Sn(s、白) △H3=+2.1kJ/mol
下列说法正确的是
[? ]
5、选择题 已知:①?C(s)?+?1/2O2(g)?==CO(g)?△H?=?–?110.5?kJ/mol? ②?C(s)+O2(g)==CO2(g)?△H=–?393.51?kJ/mol 则反应:C(s)?+?CO2(g)==2CO(g)?的△H为
[? ]
A.- 283.01?kJ/mol?
B.+?172.51?kJ/mol
C.+?283.01?kJ/mol?
D.+?504.00?kJ/mol