Кұјд:2019-06-28 21:59:58
1ЎўСЎФсМв NAҙъұн°ў·ьјУөВВЮіЈКэЎЈПВБРЛө·ЁЦРЈ¬І»ХэИ·өДКЗ
AЈ®ұкЧјЧҙҝцПВЈ¬NOәН O2ёч11.2 L»мәПЈ¬ЛщөГ»мәПЖшМеөД·ЦЧУЧЬКэОӘ 0.75 NA
BЈ®25ЎжКұЈ¬l L pH=13өДBa(OH)2ИЬТәЦРә¬УРOHЁDөДКэДҝОӘ0.1 NA
CЈ®1 mol C30H62·ЦЧУЦРә¬УР№ІјЫјьөДКэДҝОӘ 91 NA
DЈ®2.8gТТП©УлұыП©өД»мәПОпЦРә¬МјФӯЧУөДКэДҝОӘ 0.2NA
2ЎўСЎФсМв УГNAұнКҫ°ў·ьјУөВВЮіЈКэөДЦөЎЈПВБРРрКцЦРХэИ·өДКЗ
AЈ®78 g ұҪә¬УРМјМјЛ«јьөДКэДҝОӘ3NA
BЈ®іЈОВіЈС№ПВЈ¬22Ј®4 L¶юСх»ҜМјә¬УРөДФӯЧУЧЬКэОӘ3NA
CЈ®1 L 1 molЎӨL-1өДNaClO ИЬТәЦРә¬УРClOЈӯөДКэДҝОӘNA
DЈ®1 mol FeУлЧгБҝПЎHNO3·ҙУҰЈ¬ЧӘТЖ3 NAёцөзЧУ
3ЎўСЎФсМв 2molFeCl2Ул1molCl2ЗЎәГНкИ«·ҙУҰЈ¬ФтІъОпөДОпЦКөДБҝОӘ
AЈ®1mol
BЈ®2mol
CЈ®3mol
DЈ®4mol
4ЎўСЎФсМв ЙиNAОӘ°ў·ьјУөВВЮіЈКэЈ¬ПВБРРрКцЦРХэИ·өДКЗ
AЈ®ұкЧјЧҙҝцПВЈ¬22.4 LјЧұҪЦРә¬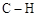 јьКэДҝОӘ8 NA
јьКэДҝОӘ8 NA
BЈ®іЈОВіЈС№ПВЈ¬7.8 gұҪЦРә¬УРЛ«јьөДКэДҝОӘ0.3 NA
CЈ®ұҪәНұҪјЧЛбөД»мәПОп1 molЈ¬НкИ«ИјЙХПыәДO2өД·ЦЧУКэОӘ7.5 NA
DЈ®іЈОВіЈС№ПВЈ¬5.2 gұҪәНұҪТТП©өД»мәПОпЦРә¬УРФӯЧУКэДҝОӘ0.8 NA
5ЎўМоҝХМв 0.5molH2OөДЦКБҝОӘ_______________Ј¬ЖдЦРә¬УР_______________ёцЛ®·ЦЧУЈ¬__________________ёцөзЧУЎЈ