Кұјд:2019-06-28 21:11:48
1ЎўСЎФсМв іЈОВПВЈ¬ПВБР¶ФУР№ШИЬТәөДЕР¶ПІ»ХэИ·өДКЗЈЁ?Ј©
AЈ®Na2CO3ИЬТәЦРЈ¬c(OH-) = c(H+)Ј«c(HCO3ЁC)Ј«c(H2CO3)
BЈ®ОВ¶ИПаН¬ЎўЕЁ¶ИПаН¬өДўЩ(NH4)2SO4ЎўўЪNaNO3ЎўўЫNH4HSO4ЎўўЬNH4NO3ЎўўЭ ЎўўЮCH3COONaИЬТәЈ¬ЛьГЗөДpHЦөУЙРЎөҪҙуөДЕЕБРЛіРтКЗЈәўЫўЩўЬўЪўЮўЭ
ЎўўЮCH3COONaИЬТәЈ¬ЛьГЗөДpHЦөУЙРЎөҪҙуөДЕЕБРЛіРтКЗЈәўЫўЩўЬўЪўЮўЭ
CЈ®NaAИЬТәөДpHЈҪ8Ј¬c(Na+)Јӯc(A-) = 0.99ЎБ10-6 mol/L
DЈ®10 mL pH = 12өДЗвСх»ҜДЖИЬТәЦРјУИлpH = 2өДHAЦБpHёХәГөИУЪ7Ј¬ЛщөГИЬТәМе»эV(ЧЬ)ЎЬ20mL
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәёщҫЭЦКЧУКШәгc(OH-) = c(H+)Ј«c(HCO3ЁC)Ј«2c(H2CO3)ҝЙЦӘЈ¬СЎПоAІ»ХэИ·ЎЈЖдУа¶јКЗХэИ·өДЈ¬ҙр°ёСЎAЎЈ
ұҫМвДС¶ИЈәјтөҘ
2ЎўСЎФсМв іЈОВКұЈ¬Ҫ«V1mL c1molЎӨLЈӯ1өД°ұЛ®өОјУөҪV2mL c2moLЎӨLЈӯ1өДСОЛбЦРЈ¬ПВБРҪбВЫЦРХэИ·өДКЗ
AЈ®Иф»мәПИЬТәөДpH=7Ј¬Фтc1V1Јҫc2V2
BЈ®ИфV1=V2Ј¬c1=c2Ј¬ФтИЬТәЦРc (NH4+) =c (Cl-)
CЈ®Иф»мәПИЬТәөДpH=7Ј¬ФтИЬТәЦРc (NH4+)Јҫc (Cl-)
DЈ®ИфV1=V2Ј¬ЗТ»мәПТәөДpHЈј7Ј¬ФтҝЙДЬУРc1 = c2
ІОҝјҙр°ёЈәAD
ұҫМвҪвОцЈә°ұЛ®КЗИхјоЈ¬ИфЗЎәГ·ҙУҰЈ¬ФтЙъіЙөДВИ»Ҝп§Л®ҪвЈ¬ИЬТәПФЛбРФЎЈИз№ыПФЦРРФЈ¬ЛөГч°ұЛ®КЗ№эБҝөДЈ¬AЎўDХэИ·Ј¬BІ»ХэИ·ЎЈёщҫЭөзәЙКШәгc (H+) +c (NH4+) =?c (Cl-)Ј«c (OH-)ҝЙЦӘЈ¬Из№ы»мәПИЬТәөДpH=7Ј¬ФтИЬТәЦРc (NH4+)ЈҪc (Cl-)Ј¬CІ»ХэИ·ЎЈҙр°ёСЎADЎЈ
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв 25ЎжЈ¬101 kPaКұЈ¬ЗҝЛбУлЗҝјоөДПЎИЬТә·ўЙъЦРәН·ҙУҰөДЦРәНИИОӘ57.3kJЎӨmol-1Ј¬РБНйөДИјЙХИИОӘ
5518 kJЎӨmol-1ЎЈПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗ
[? ]
A.2H+(aq)+SO42-(aq)+Ba2+(aq)+2OH-(aq) =BaSO4(s)+2H2O(l), ЎчH=-114.6 kJЎӨmol-1
B.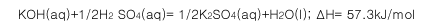
C. C8H8(l)+25/2O2(g)==8CO2+9H2O,ЎчH=-5518kJ/mol
D.2C8H18(g) +25O2(g) =16CO2(g) + 18H2O(l), ЎчH = -5518 kJЎӨmol-1
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
4ЎўСЎФсМв ПВБР№ШУЪИИ»ҜС§·ҙУҰөДГиКцЦРХэИ·өДКЗ
[? ]
AЈ®ТСЦӘH+(aq)+OH-(aq)==H2O(l)Ј»ЎчHЈҪ-57.3kJЎӨmol-1Ј¬ФтH2SO4әНBa(OH)2·ҙУҰөД·ҙУҰИИЎчH=2ЎБ(-57.3)kJЎӨmol-1
BЈ®ИјБПөзіШЦРҪ«јЧҙјХфЖшЧӘ»ҜОӘЗвЖшөДИИ»ҜС§·ҪіМКҪКЗCH3OH(g)+1/2O2(g)==CO2(g)+2H2(g)Ј» ЎчH=-192.9 kJЎӨmol-1Ј¬ФтCH3OHөДИјЙХИИОӘ192.9 kJЎӨmol-1
CЈ®H2(g)өДИјЙХИИКЗ285.8 kJЎӨmol-1Ј¬Фт2H2O(g)==2H2(g)+O2(g)Ј»ЎчH=+571.6 kJЎӨmol-1
DЈ®ЖПМСМЗөДИјЙХИИКЗ2800 kJЎӨmol-1Ј¬Фт1/2 C6H12O6 (s)+3O2 (g)==3CO2 (g)+3H2O ( l )Ј» ЎчH=-1400 kJЎӨmol-1
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв јЧНйКЗТ»ЦЦёЯР§ЗеҪаөДРВДЬФҙ,0.25 mol CH4НкИ«ИјЙХЙъіЙТәМ¬Л®Кұ·Еіц222.5 kJИИБҝ,ФтПВБРИИ»ҜС§·ҪіМКҪЦРХэИ·өДКЗ(ЎЎЎЎ)
AЈ®2CH4(g)+4O2(g) 2CO2(g)+4H2O(l)ЎЎҰӨH="+890" kJЎӨmol-1
2CO2(g)+4H2O(l)ЎЎҰӨH="+890" kJЎӨmol-1
BЈ®CH4(g)+2O2(g) CO2(g)+2H2O(l)ЎЎҰӨH="+890" kJЎӨmol-1
CO2(g)+2H2O(l)ЎЎҰӨH="+890" kJЎӨmol-1
CЈ®CH4(g)+2O2(g) CO2(g)+2H2O(l)ЎЎҰӨH="-890" kJЎӨmol-1
CO2(g)+2H2O(l)ЎЎҰӨH="-890" kJЎӨmol-1
DЈ®2CH4(g)+4O2(g) 2CO2(g)+4H2O(l)ЎЎҰӨH="-890" kJЎӨmol-1
2CO2(g)+4H2O(l)ЎЎҰӨH="-890" kJЎӨmol-1
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈәјЧНйИјЙХОӘ·ЕИИ·ҙУҰ,ЛщТФAЎўBҙнЎЈЧўТвҰӨHУл·ҪіМКҪЦРјЧНйөДОпЦКөДБҝПа¶ФУҰЎЈ
ұҫМвДС¶ИЈәТ»°г