ʱ��:2019-05-30 04:16:15
1������� ��֪�����Ȼ�ѧ����ʽ��
��1��C��s��ʯī��+O2��g���TCO2��g����H=-393.5kJ?mol-1
��2��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ?mol-1
��3��2C2H2��g��+5O2��g���T4CO2��g��+2H2O��l����H=-2599kJ?mol-1
��д��C��s��ʯī����H2��g������1mol C2H2��g�����Ȼ�ѧ����ʽ______��
2��ѡ���� ��֪101KPaʱ����ı�ȼ����Ϊ��5518kJ��mol-1����20gNaOH��ϡ��Һ��ϡ�����ַ�Ӧ�ų�����28.7kJ���������Ȼ�ѧ����ʽ��д��ȷ����
��C8H18��l��+ 25/2O2��g���� 8CO2��g��+ 9H2O��g���� ��H �� +5518 kJ��mol-1
��C8H18��l��+ 25/2O2��g���� 8CO2��g��+ 9H2O��l���� ��H �� ��5518 kJ��mol-1
��H+��aq��+ OH-��aq���� H2O��l���� ��H �� ��57��4 kJ��mol-1
��HCl (aq) + NaOH(aq) ��NaCl(aq) + H2O(l)�� ��H �� ��28.7 kJ��mol-1
A���٢�
B���ڢ�
C���ڢ�
D����
3������� (15�֣��״�����Ϊȼ�ϵ�ص�ԭ�ϡ���ҵ������CO2��H2��һ�������·�Ӧ�ϳɼ״���
��1����֪�ڳ��³�ѹ�£�
��2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H����1275.6 kJ��mol
��2CO (g)+ O2(g) �� 2CO2(g) ��H����566.0 kJ��mol
��H2O(g) �� H2O(l) ��H����44.0 kJ��mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�� ��
��2���״��������ȡ��ȩCH3OH(g) HCHO(g)+H2(g)���״���ƽ��ת�������¶ȱ仯��������ͼ��ʾ���ش��������⣺
HCHO(g)+H2(g)���״���ƽ��ת�������¶ȱ仯��������ͼ��ʾ���ش��������⣺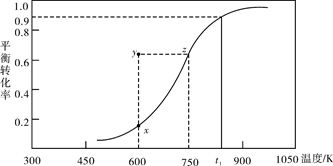
��600Kʱ��Y��״��Ħ�(��) (��)���>����<����
�ڴ�Y�㵽X��ɲ�ȡ�Ĵ�ʩ��______________________________________��
����ͬѧ����õ���t1Kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ8.1mol��L��1������Ϊ��ȷ����˵������ ��
��3������Cu2O���ھ��������Ĵ����ܶ��ܵ���ע������ͬ���ܱ������У�ʹ�ò�ͬ�����Ƶõ�Cu2O���ͣ��ֱ���д�CH3OH������ʵ�飺CH3OH(g) HCHO(g)+H2(g)
HCHO(g)+H2(g)
CH3OH��Ũ�ȣ�mol��L��1����ʱ��t (min)�仯���±���
| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
4��ѡ���� ��CH4����ԭNOx�������������������Ⱦ����֪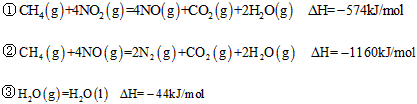
����˵������ȷ����
[? ]
A�������ʵ�����CH4�μӷ�Ӧ����Ӧ�٢�ת�Ƶĵ�������ͬ
B��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(l);�SH=-618KJ/mol
C��0��2 mol CH4��ԭNO2��N2��������H2O(g)�ų�������Ϊ173��4kJ
D������4��48 L(��״��)CH4��ԭNO2��N2������������ת�Ƶ���1��60mol
5������� ��6�֣����ף�P���Ͱ��ף�P4����Ϊ��ͬ�������塣��֪��
P4 ( ��s )�� 5O2 ( g )�� P4O10( s ) ��H =" -2983.2" kJ/mol
P(�� s )�� 5/4O2 ( g )�� 1/4P4O10( s ) ��H =" -738.5" kJ/mol
д������ת��Ϊ�����Ȼ�ѧ����ʽ ,�ɴ˿�֪�����ױȰ��� ������ȶ������ȶ�����