ʱ��:2019-05-30 04:02:12
1��ѡ���� ��-AgI��һ�ֹ��嵼�壬�����ʺܸߣ�Ϊ�о���-AgI������Ag+���绹��I-���磬�������ͼ��ʾʵ�飮�����й�˵������ȷ���ǣ�������
A��������Ӧ��Ag-e-�TAg+
B������������������
C������-AgI��Ag+���磬��ͨ��һ��ʱ�������������
D������-AgI��Ag+���磬��ͨ��һ��ʱ�����������С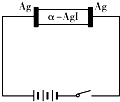
�ο��𰸣����������������������������缫��ӦΪAg-e-�TAg+�������缫��ӦΪAg++e-�TAg�����-AgI��Ag+���磬��ͨ��һ��ʱ������������䣬���ǵ����ӵ��磬�����ܶȼ�С��
A�������缫��ӦΪAg-e-�TAg+����A��ȷ��
B�������缫��ӦΪ��Ag++e-�TAg������������������B��ȷ��
C������-AgI��Ag+���磬�͵��ԭ�����ƣ���ͨ��һ��ʱ������������䣬��C��ȷ��
D������-AgI��Ag+���磬�͵��ԭ�����ƣ���ͨ��һ��ʱ������������䣬��D����
��ѡD��
���������
�����Ѷȣ���
2��ѡ���� �ö��Ե缫���һ����������ͭ��Һ����ת��0.4 mol����ʱ��������ʼ�������壬���������ת��0.2 mol����ʱֹͣ��⡣����˵����ȷ����
A���������У���Һ��pH��������
B����ת��0.4 mol ����ʱ��������ɵ�ͭΪ12.8 g
C��������ӦʽΪ2H2O��4e��=4H����O2��
D�����������в�����H2��O2�������Ϊ2:1
�ο��𰸣�B
���������A.�����̷����Σ���һ�ε������ͭ��Һ����Һ�����ԣ��ڶ��Σ�ʵ�����ڵ��ˮ��������ǿ������B.��һ��2CuSO4 +2H2O 2Cu��+O2��+2H2SO4ת��4e������ת��0.4 mol ����ʱ��������ɵ�ͭΪ12.8 g ����ȷ��C.���������̷����Σ�������ӦʽΪCu+2e��=Cu��2H��+2e��=H2��������D.��һ��2CuSO4 +2H2O
2Cu��+O2��+2H2SO4ת��4e������ת��0.4 mol ����ʱ��������ɵ�ͭΪ12.8 g ����ȷ��C.���������̷����Σ�������ӦʽΪCu+2e��=Cu��2H��+2e��=H2��������D.��һ��2CuSO4 +2H2O 2Cu��+O2��+2H2SO4ת��4e��
2Cu��+O2��+2H2SO4ת��4e��
0.1 0.4
�ڶ��Σ�2H2O 2H2��+O2��ת��4e��
2H2��+O2��ת��4e��
0.1 0.05 0.1 ���������в����������������������Ϊ2:3������ѡB��
���㣺������ԭ����Ӧ�á�
�����Ѷȣ�һ��
3��ѡ���� ��ͼ�ǵ��CuCl2��Һ��װ�ã�����c��dΪʯī�缫���������йص��ж���ȷ���� 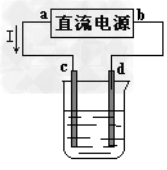
[? ]
A��a������b����?
B��a������b����
C���������У�d�缫��������?
D���������У�������Ũ�Ȳ���
�ο��𰸣�C
���������
�����Ѷȣ�һ��
4��ѡ���� ��X������Y���Z��ˮ��Һ�����һ��ʱ����ټ���W����ʹ��Һ�ָ������ǰ��״̬�����±����������һ���ǣ�������
| ��� | X | Y | Z | W A C Fe NaCl H2O B Pt Cu CuSO4 CuSO4��Һ C C C H2SO4 H2O D Ag Fe AgNO3 AgNO3��Һ |
�ο��𰸣�A����Fe��CΪ�缫������Ȼ��ƣ����������ӷŵ磬���������ӷŵ磬ͨ��һ��ʱ���Ϊ����������Һ���������Ȼ��غ�ˮ����A����
B����Pt��CuΪ�缫���������ͭ���������������ӷŵ磬����ͭ���ӷŵ磬ͨ��һ��ʱ���Ϊ������Һ����Ҫ��������ͭ��̼��ͭ�ָ��Ѷȣ���B����
C����CΪ�缫��������ᣬ�������������ӷŵ磬���������ӷŵ磬�൱�ڵ��ˮ��ͨ��һ��ʱ�����Ϊ������Һ�����Լ�ˮ����Һ��ԭ����C��ȷ��
D����Ag��FeΪ�缫�����AgNO3�����������ӷŵ磬���������ӷŵ磬�൱�ڵ�ƣ�ͨ��һ��ʱ�����ΪAgNO3��Һ����Ũ�Ȳ��䣬����Ҫ��AgNO3����ָ�ԭ������D����
��ѡC��
���������
�����Ѷȣ�һ��
5��ѡ���� ��ͼ��װ�ý���ʵ�飬��ͼ���к�����X��ʾͨ���缫�ĵ��ӵ����ʵ���������������ȷ����
[? ]
A��F��ʾ��Ӧ����Cu�����ʵ���
B��E��ʾ��Ӧ����H2O�����ʵ���
C��E��ʾ��Ӧ����O2�����ʵ���
D��F��ʾ��Ӧ����H2SO4�����ʵ���
�ο��𰸣�B
���������
�����Ѷȣ�һ��