时间:2018-03-17 05:16:29
1、填空题 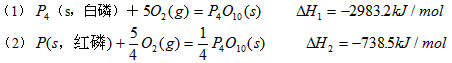
则白磷转化为红磷的热化学方程式_______________________ 。相同的状况下,能量较低的是_______;白磷的稳定性比红磷___________(填“高”或“低”)。
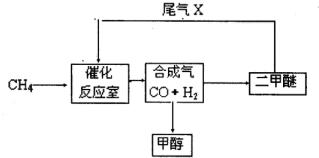
2、简答题 合成气(CO+H2)是一种重要的化工原料,在化工生产中具有十分广泛的用途.?可以生产被称为21世纪的新型燃料--甲醇、二甲醚(CH3OCH3)等物质.其工艺流程如下:
(1)写出用合成气生产二甲醚的化学方程式______.
(2)尾气循环中涉及到以下反应:CH4(g)+H2O(g)?CO(g)+3H2(g),请写出某温度下该反应的平衡常数表达式______.
(3)工业上一般采用下列两种反应合成甲醇:
反应Ⅰ:CO(g)+2H2(g)?CH3OH(g)△H1
反应Ⅱ:CO2(g)+3H2(g)?CH3(g)+H2O(g)△H2
①下表所列数据是反应在不同温度下的化学平衡常数(K).
| 温度 | 250 | 300 | 350 K 2.041 0.270 0.012 |
| 1 2 |
| 1 2 |
3、简答题 1840年盖斯根据一系列实验事实得出规律,他指出:“若是一个反应可以分步进行,则各步反应的吸收或放出的热量总和与这个反应一次发生时吸收或放出的热量相同.”这是18世纪发现的一条重要规律,称为盖斯定律.已知1mol?金刚石和石墨分别在氧气中完全燃烧时放出的热量为:金刚石,395.41kJ;石墨,393.51kJ.则金刚石转化石墨时,放热还是吸热?______,其数值是_______,由此看来更稳定的是______.若取金刚石和石墨混合晶体共1mol?在O2中完全燃烧,产生热量为QkJ,则金刚石和石墨的物质的量之比为______(用含Q的代数式表示).
4、选择题 己知?25℃、101kPa条件下:
4Al(s)+3O2(g)=2Al2O3,△H=-2834.9kJ?mol-1
4Al(s)+2O3(g)=2Al2O3,△H=-3119.1gkJ?mol-1
由此得出的正确结论是( )
A.等质量的O2比O3的能量低,由O2变O3为放热反应
B.等质量的O2比O3的能量低,由O2变O3为吸热反应
C.O3比O2稳定,由O2变O3为放热反应
D.O2比O3稳定,由O2变O3为吸热反应
5、简答题 硝酸是一种重要的化工原料,工业上一般以氨气为原料来制备硝酸.请回答:
(1)氨气催化氧化的化学方程式为______.
(2)硝酸厂的尾气直接排放将污染空气,目前科学家探索利用燃料气体中的甲烷等将氮的氧化物还原为氮气和水,反应机理为:
CH4(g)+4NO2(g)═4NO(g)+CO2(g)+2H2O(g)△H=-574kJ?mol-1
CH4(g)+4NO(g)═2N2(g)+CO2(g)+2H2O(g)△H=-1160kJ?mol-1
则甲烷直接将NO2还原为N2的热化学方程式为:______.
(3)氨气若在纯氧中燃烧,则发生反应为4NH3+3O2
? |