ʱ��:2017-11-05 22:59:50
1��ѡ���� ��0.01mol?L-1��NaHCO3��Һ�У����б�����ȷ���ǣ�������
A����Һ�����ԣ�HCO3-����̶ȴ���ˮ��̶�
B��[Na+]+[H+]=[HCO3-]+[CO32-]+[OH-]
C��[Na+]=[HCO3-]+[CO32-]+[H2CO3]
D����Һ���еĵ������ֻ��NaHCO3�TNa++HCO3-
�ο��𰸣�A����Һ�Լ��ԣ�HCO3-����̶�С��ˮ��̶ȣ���A����
B���ɵ���غ��֪��[Na+]+[H+]=[HCO3-]+2[CO32-]+[OH-]����B����
C���������غ��֪��[Na+]=[HCO3-]+[CO32-]+[H2CO3]����C��ȷ��
D����Һ���еĵ���������εĵ��롢̼��������ӵĵ��롢ˮ�ĵ��룬��D����
��ѡC��
���������
�����Ѷȣ���
2��ѡ���� ���и���Һ�У������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
A��0.1mol?L-1Na2CO3��Һ�У�[OH-]=[HCO3]+[H+]��[C
�ο��𰸣�A��̼������Һ�У����������غ�ã�C��Na+��=2C��HCO3-��+2C��CO32-��+2C��H2CO3������Һ�ʵ����ԣ���Һ���������������ȣ�C��Na+��+C��H+��=C��HCO3-��+2C��CO32-��+C��OH-������������ʽ��C��OH-��=C��HCO3-��+2C��H2CO3����̼������ˮ��Һ���Ե���Ϊ����ˮ��Ϊ�Σ�����C��CO32-����C��OH-������A����
B������炙�ѧʽ�У�笠����Ӻ���������ӵĸ�����=2��1��ˮ��Һ��笠�����ˮ�����Һ��C��H+����C��OH-������ˮ�������ģ�����C��NH4+��C��SO42-������B����
C��̼���������ˮ���C��Na+����C��HCO3-����C��H+����C��OH-������̼��������ӵ�ˮ�������ģ�����C��HCO3-����C��OH-������C����
D�����ƻ�ѧʽ�������Ӻ������Ӹ�������2��1��������Һ�и��������غ��C��Na+��=2C��HS-��+2C��S2-��+2C��H2S������D��ȷ��
��ѡD��
���������
�����Ѷȣ���
3��ѡ���� һ���¶��£���Cl2����ͨ��ˮ�������ͣ�Ȼ�������ñ�����ˮ�еμ�0.1mol/L��NaOH��Һ����ҺpH�仯��������ͼ��ʾ��������������ȷ����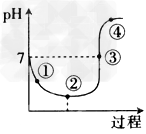
[? ]
A.�����ʾ��Һ�У�c(H+)=c(Cl-)+c(HClO)+c(OH-)
B.�����ʾ��Һ�У�c(H+)>?c(Cl-)>c(ClO-)>c(HClO)
C.�����ʾ��Һ�У�c(Na+)=2c(ClO-)+c(HClO)
D.�����ʾ��Һ�У�c(Na+)>c(ClO-)>c(Cl-)>c(HClO)
�ο��𰸣�C
���������
�����Ѷȣ�һ��
4��ѡ���� �����ΪVa��0.05mol?L-1CH3COOH��Һ�м������ΪVb��0.05mol?L-1KOH��Һ�����й�ϵ������ǣ�������
A��Va��Vbʱ��c��CH3COOH��+c��CH3COO-����c��K+��
B��Va=Vbʱ��c��CH3COOH��+c��H+��=c��OH-��
C��Va��Vbʱ��c��CH3COO-����c��K+����c��OH-����c��H+��
D��Va��Vb�����ʱ��c��K+��+c��H+��=c��OH-��+c��CH3COO-��
�ο��𰸣�A����ͼ�����ʵ���Ũ����ȣ��Ҷ���һԪ�ģ���Va��Vbʱ�����������Һ�е������Ǵ���غʹ��ᣬ���������غ�֪��c��CH3COOH��+c��CH3COO-����0.025mol/L��c��K+����0.025mol/L������c��CH3COOH��+c��CH3COO-����c��K+������A��ȷ��
B����Va=Vbʱ����ͼ�ǡ���кͣ���Һ�е������Ǵ���أ����������غ�֪��c��CH3COOH��+c��H+��=c��OH-������B��ȷ��
C����Va��Vbʱ���������ع�������Һ�е������Ǵ���غ��������أ���Һ�м�����Ũ�ȴ��ڴ��������Ũ�ȣ���Һ�ʼ��ԣ���������������Ũ�ȴ���������Ũ�ȣ����������ص����ԶԶ���ڴ���������������������Ũ�ȴ��ڴ��������Ũ�ȣ���������������ȴ����������ʱ�����������Ũ�ȴ�������������Ũ�ȣ���C����
D��Va��VbΪ�����ʱ����Һ�ʵ����ԣ���Һ�������������������ȣ����ݵ���غ�ã�c��K+��+c��H+��=c��OH-��+c��CH3COO-������D��ȷ��
��ѡC��
���������
�����Ѷȣ�һ��
5��ѡ���� �����±��ṩ�����ݣ��ж��ڵ�Ũ�ȵ�NaCN��NaHS�Ļ����Һ�У���������Ũ�ȹ�ϵ��ȷ����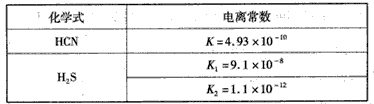
[? ]
A.c(CN -)>c(HS -)>c(OH -)
B.c(HS -)>c(CN -)>c(OH -)
C.c(Na+)+c(H+)=c(HS -)+c(CN-)+c(OH -)
D.c( HCN) +c(CN -)=c(HS -)+c( H2S)
�ο��𰸣�B
���������
�����Ѷȣ�һ��