ʱ��:2017-09-24 01:06:16
1��ѡ���� ���л�ѧ������д��ȷ���ǣ�������
A�������Ļ�ѧʽΪKAlSO4?12H2O
B�������ӵĽṹʾ��ͼΪ��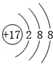
C������ĵ��뷽��ʽ��H2SO4�TH2++SO42-
D������Ļ�ѧʽ��NaCO3
2������� ��A��B��C��D���ֶ���������Ԫ�أ����ǵ�ԭ������������������AԪ��ԭ�Ӻ�����ӽ���һ��ԭ�ӹ����Ҳ����������ḻ��Ԫ�أ�BԪ��ԭ�ӵĺ���p��������s��������1��CΪ����Ԫ����ԭ�Ӻ���p��������s��������ȣ�DԪ�ص�ԭ�Ӻ�������p���ȫ���������
��1��д������Ԫ�ص�Ԫ�ط��ţ�A______��B______��C______��D______��
��2��д��C��D����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ͼ��C______D______
��3��д��B��C����Ԫ�ص�����һ�������·�Ӧ�Ļ�ѧ����ʽ��______��
��4��д��BԪ�ص��ʺ��⻯��ĵ���ʽ������______���⻯��______��
3������� ���ڱ���ǰ�������е�����Ԫ��A��B��C��D��E��Fԭ����������������֪Aԭ��2p�����3��δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ����۵���ͬ�����γɵĵ���������ߵģ�F2����������Ӳ���Ӿ��ѳ�����
����������Ϣ�ش��������⣺
(1)д��Dԭ�Ӻ�������Ų�ʽ��____________________________________________��
(2)A��B��C��D�ĵ�һ��������С�����˳��Ϊ________________________________(��Ԫ�ط��ű�ʾ)��
(3)B���Ȼ�����۵��D���Ȼ�����۵�________(��ߡ��͡�)��������_______________________________________________________��
(4)E�������������ӵĿռ乹����________����________(����ԡ��Ǽ��ԡ�)���ӡ�
(5)E��F�γɵ�ij�ֻ���������ͼ��ʾ�ľ���ṹ���û����ﻯѧʽΪ________��Eԭ����λ��Ϊ________________________________________________��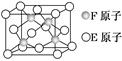
4��ѡ���� �����������ճ�����������Ҫ�ij������ִ����ڡ�ú���͡������⻯ѧ��������Ⱦ�����ڽ����ԡ����ڿ�����Ⱦ��Ϊ��־�ĵ�����Ⱦʱ�ڡ�������ڻ�����Ⱦ���ж�������Ҫ�м�ȩ��������������֡�
��ش��������⣺
��1�������ӵĵ���ʽ��? ?�������ӵĿռ乹��Ϊ? ?��
��2������������ڿ�����Ⱦ��һ����ʩ��? ?��
��3���ҹ������ڿ������������涨���ڿ����м�ȩ�������ó���0.08mg?m-3����ȩ�ⶨ�ķ�����Ҫ�зֹ��ȷ���ɫ�����绯ѧ������ѧ�ζ����ȡ�ijУ�о���ѧϰС���ͬѧ����û�ѧ�ζ����Խ����ڿ����м�ȩ�ĺ������вⶨ������������о����⣬��ɼ���ȩ��Ʒ�ķ�����? ?��
��4���������ǻ�ѧ�ζ���֮һ��������ԭ��Ϊ�ڼ��Խ��ʣ�NaOH���У���ת��Ϊ�ε����ƺ͵⻯�ƣ��ε����ƽ���Һ������ļ�ȩ����Ϊ�����ƣ����ʵ��ữ��ʣ��Ĵε�������⻯�������ɵ⣻�Ե���Ϊָʾ��������������Ʊ���Һ�ζ��������������漰�Ļ�ѧ��Ӧ�����ӷ���ʽ����ΪI2+2OH-====IO-+I-+H2O��
? ?��? ?��I2+2S2O32-====S4O62-+2I-��ʵ���������Ҫ�ⶨ�����ݳ��ܵ����⣬����? ?��
5������� ��Ԫ�ؿ����γɶ���������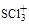 ��SO2��SO32-��SO42-�ȡ�
��SO2��SO32-��SO42-�ȡ�
��1��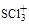 ��Sԭ�ӵĹ���ӻ�������?��
��Sԭ�ӵĹ���ӻ�������?��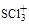 �Ŀռ乹����?��
�Ŀռ乹����?��
��2����[Cu(NH3)4]SO4��Һ��ͨ��SO2�����ԣ��а�ɫ�������ɡ����������ó�����Cu��S��N�����ʵ���֮��Ϊ1:1:1�����ⶨ�ó����ľ�������һ�������͵������Ӻ�һ�����������͵������ӡ�
��[Cu(NH3)4]SO4��Cu2+�ĵ����Ų�ʽΪ?��
��[Cu(NH3)4]SO4�д��ڵĻ�ѧ��������?������ţ���
A�����ۼ�
B�����
C�����Ӽ�
D����λ�� E�����Ӽ�������
��д����SO42-��Ϊ�ȵ������һ�ַ���?��������ɫ�����Ļ�ѧʽΪ?��