ʱ��:2017-09-23 22:35:46
1��ѡ���� ���и���Ӧ�У�ˮ����ԭ������
A.2H2+O22H2O
B.2F2+2H2O�T4HF+O2
C.SO3+H2O�TH2SO4
D.2Na+2H2O�T2NaOH+H2��
�ο��𰸣�B
�����������������ˮ����ԭ������ˮ��OԪ�صĻ��ϼ����ߣ��Դ������
���A.2H2+O2 2H2O�У�ˮΪ�������ˮ���ǻ�ԭ������A����
2H2O�У�ˮΪ�������ˮ���ǻ�ԭ������A����
B.2F2+2H2O�T4HF+O2�У�ˮ��OԪ�صĻ��ϼ����ߣ���ˮ����ԭ������B��ȷ��
C��SO3+H2O�TH2SO4�У���Ԫ�صĻ��ϼ�û�б仯��������������ԭ��Ӧ��ˮΪ��Ӧ���C����
D.2Na+2H2O�T2NaOH+H2���У�ˮ��HԪ�صĻ��ϼ۽��ͣ���ˮΪ����������D����
��ѡB��
���������⿼��������ԭ��Ӧ����ȷ��Ӧ��Ԫ�صĻ��ϼ۱仯����ԭ�����жϼ��ɽ����Ŀ�ϼ�
�����Ѷȣ�һ��
2������� ��12�֣�2SO2(g)��O2(g) 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ��
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ��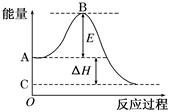
��֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol����ش��������⣺
��ͼ��A���ʾ�� _____��C���ʾ�� (���Ӧ����������������������������)
��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ��________(��С����ޡ�)Ӱ�졣
��ͼ�Ц�H��________kJ/mol��
�ο��𰸣���1����Ӧ��������������������������2���� ��3����198
���������(1)��С�⿼�鷴Ӧ������������������������Ϊ���ȷ�Ӧ���ɵõ�A��C����ʾ�����塣
��2��EΪ����뷴Ӧ���أ������ô������Խ��ͻ�ܡ�
��3��ͼ�б�ʾ����2molSO2�ķ�Ӧ����ˡ�H����99��2KJ��mol-1����198kJ/mol
�����Ѷȣ�һ��
3��ѡ���� ������ʵ�У��ܹ�֤��HCl�ǹ��ۻ��������
A��HCl������ˮ
B��Һ̬��HCl������
C��HCl���ֽ�
D��HCl����ˮ�ܵ��룬������
�ο��𰸣�B
������������ۻ�����Ĺ����������磬��Һ̬�����硣�����ӻ�����Ĺ��������磬��Һ̬�ܵ��磬�Դ���֤���Ȼ����ǹ��ۻ����
A�����������ܽ����ж��Ƿ�Ϊ���ۻ������NaClҲ������ˮ�����������ӻ������A����
B���ۻ�����Ĺ����������磬���ӻ�����Ĺ��������磬�������Һ̬�Ȼ��ⲻ������֤���Ȼ����ǹ��ۻ������B��ȷ��
C���������÷ֽ����ж��Ƿ�Ϊ���ۻ������NaCl����Ҳ���ֽ⣬���������ӻ������C����
D���Ȼ�������ˮ����Һ�����ԣ�������Ϊ�ж��Ƿ�Ϊ���ۻ���������ݣ�����������������ˮҲ�����ԣ����������������ӻ������D����ѡB��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά����������˼ά��������ȷ���ۻ�����Ĺ������ǽ����Ĺؼ���
�����Ѷȣ���
4������� �±�ΪԪ�����ڱ���һ���֣�
| �� ���� | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |

�ο��𰸣���1��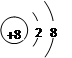 ��1�֣� ��2��S2-��O2-�� Na+��2�֣� ��3��SiCl4
��1�֣� ��2��S2-��O2-�� Na+��2�֣� ��3��SiCl4
��4��2Na2O2 +2H2O��4Na+ + 4OH
���������
�����Ѷȣ�����
5��ѡ���� ��֪���ȼ��a g��Ȳ��C2H2������ʱ����2 mol������̼�����Һ̬ˮ�����ų�����b kJ������Ȳȼ���ȵ��Ȼ�ѧ����ʽ��ȷ����( )
A��2C2H2��g����5O2��g����4C��Դ:91������ 91Exam.orgO2��g����2H2O��l�� ��H����2b kJ / mol
B��C2H2��g����5/2O2��g����2CO2��g����H2O��l�� ��H����b kJ / mol
C��2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H����4b kJ / mol
D��2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H��b kJ / mol
�ο��𰸣�B
������������ȼ��a g��Ȳ����ʱ����2mol������̼�����Һ̬ˮ�����ų�����b kJ�������ʵ����ʵ����뷴Ӧ�ų������������ȿ�֪������4mol������̼�����Һ̬ˮ�����ų�����2bkJ����ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų����������ɱ�ʾΪC2H2��g��+5/2O2��g��=2CO2��g��+H2O��l����H=-bkJ/mol,ѡB��
���㣺����ȼ���ȵļ���
�����Ѷȣ�һ��