±Љд:2017-08-26 00:41:07
1°Ґ—°‘сћв ∞—pH=2µƒH2SO4ЇЌpH=11µƒNaOH»№“ЇїмЇЌ£ђїмЇЌ“ЇpH=7°£‘тЅљ»№“Їµƒћеїэ±» «£®?£©
A£Ѓ10:1
B£Ѓ1:10
C£Ѓ1:2
D£Ѓ2:1
≤ќњЉір∞Є£ЇB
±Њћвљвќц£ЇїмЇѕЇу»№“ЇµƒpH£љ7£ђЋщ“‘«вјл„”µƒќп÷ µƒЅњµ»”ЏOH£≠µƒќп÷ µƒЅњ£ђЉі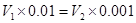 £ђљвµ√
£ђљвµ√ ©U
©U £љ1:10£ђір∞Є—°B°£
£љ1:10£ђір∞Є—°B°£
µг∆ј£Ї‘ЏЉ∆Ћг»№“ЇµƒpH ±£ђ»зєы «ЋбЇЌЉоЈі”¶£ђ‘т „ѕ»“™≈–ґѕЋбЇЌЉоµƒєэЅњќ ћв£ђ»зєыЋбєэЅњ£ђ‘т „ѕ»Љ∆Ћг»№“Ї÷–«вјл„”≈®ґ»°£»зєы «ЉоєэЅњ£ђ‘т „ѕ»Љ∆Ћгµƒ «»№“Ї÷–OH£≠µƒ≈®ґ»£ђ»їЇуЄщЊЁЋЃµƒјл„”їэ≥£ э‘ЏїїЋг≥…«вјл„”≈®ґ»°£
±Њћвƒ—ґ»£Ї“ї∞г
2°Ґ—°‘сћв ”√≤ђ„чµзЉЂµзљв500 mLЇђKNO3ЇЌCu£®NO3£©2µƒїмЇѕ»№“Ї“їґќ ±ЉдЇу£ђ‘ЏЅљЉЂЊщ…ъ≥…11£Ѓ2L±к„Љ„іњцѕ¬µƒ∆шће£ђ‘≠»№“Ї÷–Cu2+µƒќп÷ µƒЅњ≈®ґ»ќ™£®?£©
A£Ѓ0£Ѓ5 mol£ѓL
B£Ѓ2 mol£ѓL
C£Ѓl mol£ѓL
D£ЃќёЈ®»Јґ®
≤ќњЉір∞Є£ЇC
±Њћвљвќц£Ї
’э»Јір∞Є£ЇC
Є√єэ≥ћЅЋ…ъЅљЄцЈі”¶£ђѕ»µзљвCu (NO3)2»№“Ї£ђЇуµзљвЋЃ
2H2O 2H2°ь?£Ђ? O2°ь
2H2°ь?£Ђ? O2°ь
0.5mol? 0.25mol
2Cu(NO3)2£Ђ2H2O 2Cu£ЂO2°ь£Ђ4HNO3
2Cu£ЂO2°ь£Ђ4HNO3
0.5mol? 0.5mol®D0.25mol
C(Cu2£Ђ)=0.5mol/0.5?L=l mol£ѓL
±Њћвƒ—ґ»£ЇЉтµ•
3°Ґ—°‘сћв ѕ¬Ѕ–ЋЃ»№“Ї“їґ®≥ ÷––‘µƒ «
A£ЃpH=7µƒ»№“Ї
B£Ѓc(H+)=1.0°Ѕ10-7mol°§L-1µƒ»№“Ї
C£Ѓc(H+) =c(OH-)
D£ЃpH=3µƒ”лpH=11µƒЉоµ»ћеїэїмЇѕЇуµƒ»№“Ї
≤ќњЉір∞Є£ЇC
±Њћвљвќц£Ї»№“ЇµƒЋбЉо–‘ «”…c(H+)”лc(OH-)µƒѕаґ‘іу–°Њцґ®µƒ
±Њћвƒ—ґ»£ЇЉтµ•
4°Ґ—°‘сћв ѕ¬Ѕ–»№“Ї÷–µƒЅ£„”≈®ґ»єЎѕµ’э»Јµƒ «
A£Ѓ0.1 mol/L NaHCO3»№“Ї÷–£Їc(Na+)£Њc(HCO3-)£Њc(CO32-)£Њc(H2CO3)
B£Ѓ1L0.1 mol/L Na2S»№“Ї÷–£Їc(OH-)-c(H+)£љc(HS-)+c(H2S)
C£Ѓ “ќ¬ѕ¬£ђpH£љ3.5µƒЄћйў÷≠÷–c(H+) «pH£љ6.5µƒ≈£ƒћ÷–c(H+)µƒ1000±ґ
D£Ѓµ»ћеїэ°Ґµ»ќп÷ µƒЅњ≈®ґ»µƒNaXЇЌ»хЋбHXїмЇѕЇуµƒ»№“Ї÷–£Ї
c(Na+)£Њc(HX)£Њc(X-)£Њc(H+)£Њc(OH-)
≤ќњЉір∞Є£ЇC
±Њћвљвќц£ЇA°ҐћЉЋб«вƒ∆»№”ЏЋЃ£ђЋЃљв≥ћґ»іу”Џµзјл≥ћґ»£ђ»№“Їѕ‘Љо–‘£ђ‘тc(Na+)£Њc(HCO3-)£Њc(H2CO3) £Њc(CO32-)£ђA≤ї’э»Ј£їB°ҐЄщЊЁ÷ „” ЎЇгњ…÷™£ђЅтїѓƒ∆»№“Ї÷–c(OH-)£љc(H+)+c(HS-)+2c(H2S)£ђB≤ї’э»Ј£їC°Ґ “ќ¬ѕ¬£ђpH£љ3.5µƒЄћйў÷≠÷–c(H+) «10£≠3.5mol/L£ђpH£љ6.5µƒ≈£ƒћ÷–c(H+) «10£≠6.5mol/L£ђ“тіЋ«∞’я «Їу’яµƒ1000±ґ£ђC’э»Ј£їD°Ґµ»ћеїэ°Ґµ»ќп÷ µƒЅњ≈®ґ»µƒNaXЇЌ»хЋбHXїмЇѕЇуµƒ»№“Ї÷–ЋЃљв≥ћґ»іу”Џµзјл≥ћґ»£ђ»№“Їѕ‘Љо–‘£ђ“тіЋc(HX)£Њc(Na+)£Њc(X-)£Њc(OH-)£Њc(H+)£ђD≤ї’э»Ј£ђір∞Є—°C°£
±Њћвƒ—ґ»£Ї“ї∞г
5°Ґ—°‘сћв ѕ¬Ѕ–»№“Ї÷–µƒ¬»јл„” эƒњ”л50 mL 1 mol°§L£≠1µƒFeCl3»№“Ї÷–¬»јл„” эƒњѕаµ»µƒ «£®?£©
A£Ѓ50 mL 1.5 mol°§L£≠1µƒFeCl2»№“Ї
B£Ѓ100 mL 3 mol°§L£≠1µƒNH4Cl»№“Ї
C£Ѓ75 mL 3 mol°§L£≠1µƒKCl»№“Ї
D£Ѓ50 mL 2 mol°§L£≠1µƒCaCl2»№“Ї
≤ќњЉір∞Є£ЇA
±Њћвљвќц£ЇЄщЊЁ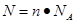 њ…÷™£ђ¬»јл„” эƒњѕаµ»£ђ‘т¬»јл„”µƒќп÷ µƒЅњ“≤ «ѕаµ»µƒ°£50 mL 1 mol°§L£≠1µƒFeCl3»№“Ї÷–¬»јл„”µƒќп÷ µƒЅњ «0.05L°Ѕ1mol/L°Ѕ3£љ0.15mol£ђґш—°ѕо÷–¬»јл„”µƒќп÷ µƒЅњЈ÷±р «0.15mol°Ґ0.3mol°Ґ0.225mol°Ґ0.2mol£ђЋщ“‘ір∞Є—°A°£
њ…÷™£ђ¬»јл„” эƒњѕаµ»£ђ‘т¬»јл„”µƒќп÷ µƒЅњ“≤ «ѕаµ»µƒ°£50 mL 1 mol°§L£≠1µƒFeCl3»№“Ї÷–¬»јл„”µƒќп÷ µƒЅњ «0.05L°Ѕ1mol/L°Ѕ3£љ0.15mol£ђґш—°ѕо÷–¬»јл„”µƒќп÷ µƒЅњЈ÷±р «0.15mol°Ґ0.3mol°Ґ0.225mol°Ґ0.2mol£ђЋщ“‘ір∞Є—°A°£
µг∆ј£ЇЄ√ћв «їщі°–‘ ‘ћвµƒњЉ≤й£ђ“≤ «ЄяњЉ÷–µƒ≥£ЉыњЉµг°£ ‘ћвїщі°–‘«њ£ђ÷ч“™ «њЉ≤й—І…ъґ‘“‘ќп÷ µƒЅњќ™ЇЋ–ƒµƒ”–єЎЉ∆Ћгµƒ мѕ§’∆ќ’≥ћґ»°£Є√ћвµƒєЎЉь «√ч»Ј»№“Ї÷–јл„”µƒ≈®ґ»”лќп÷ ≈®ґ»µƒєЎѕµ£ђћЎ±р «“™„Ґ“в£ђ“™«уЉ∆Ћгµƒ «≈®ґ»їє «ќп÷ µƒЅњ°£
±Њћвƒ—ґ»£ЇЉтµ•