К±јд:2019-03-14 22:45:51
1ЎўСЎФсМв КµСйКТУГKMnO4ИЬТєµО¶ЁДЬЅшРРГїЖ¬І№СЄјБє¬МъФЄЛШЈЁFe2+Ј©є¬БїµДІв¶ЁЈ¬»ЇС§ФАнОЄЈє5Fe2++MnO4-+8H+ЁT5Fe3++Mn2++4H2OЈ®ПВБРµО¶Ё·ЅКЅЦРЈЁјРіЦІї·ЦВФИҐЈ©Ј¬ЧоєПАнµДКЗЈЁ? Ј©
AЈ®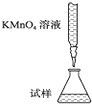
BЈ®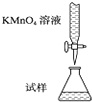
CЈ®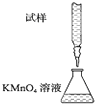
DЈ®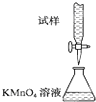
2ЎўКµСйМв ФЪЦРєНµО¶ЁІЩЧч№эіМЦРЈ¬УРТФПВёчПоТтІЩЧчІ»µ±ТэЖрµДКµСйОуІоЈ¬УГЎ°Ж«ёЯЎ±Ў°Ж«µНЎ±»тЎ°ОЮУ°ПмЎ±µИМоїХЈє
(1)µО¶Ё№ЬУГХфБуЛ®Пґѕ»єуЈ¬ОґУГТСЦЄЕЁ¶ИµД±кЧјИЬТєИуПґЈ¬К№µО¶ЁЅб№ы___Ј»
(2)Ч¶РОЖїУГХфБуЛ®Пґѕ»єуЈ¬УЦУГґэІвИЬТєИуПґЈ¬К№µО¶ЁЅб№ы____Ј»
(3)µО¶Ё№ЬЈЁЧ°±кЧјИЬТєЈ©ФЪµО¶ЁЗ°јвЧ촦УРЖшЕЭЈ¬µО¶ЁЦХБЛОЮЖшЕЭЈ¬К№µО¶ЁЅб№ы____Ј»
(4)µО¶ЁЗ°ЖЅКУЈ¬µО¶ЁЦХБЛё©КУЈ¬К№µО¶ЁЅб№ы____Ј»
(5)µО¶ЁЗ°СцКУЈ¬µО¶ЁЦХБЛЖЅКУЈ¬К№µО¶ЁЅб№ы____Ј»
(6)№эФз№АјЖЦХµгЈ¬К№µО¶ЁЅб№ы____Ј»
(7)№эНн№АјЖЦХµгЈ¬К№µО¶ЁЅб№ы____ЎЈ
3ЎўКµСйМв КµСйКТЦРУРТ»ОґЦЄЕЁ¶ИµДПЎСОЛбЈ¬ДіС§ЙъОЄІв¶ЁСОЛбµДЕЁ¶ИФЪКµСйКТЦРЅшРРИзПВКµСйЎЈ
ЗлНкіЙПВБРМоїХЈє
ўЩЕдЦЖ100mL 0Ј®10mol/L NaOH±кЧјИЬТєЎЈ
ўЪИЎ20Ј®00mLґэІвПЎСОЛбИЬТє·ЕИлЧ¶РОЖїЦРЈ¬ІўµОјУ2~3µО·УМЄЧчЦёКѕјБЈ¬УГЧФјєЕдЦЖµД±кЧјNaOHИЬТєЅшРРµО¶ЁЎЈЦШёґЙПКцµО¶ЁІЩЧч2~3 ґОЈ¬јЗВјКэѕЭИзПВЎЈ 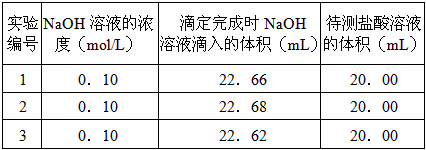
ЈЁ1Ј©јоКЅµО¶Ё№ЬУГХфБуЛ®Пґѕ»єуФЪЧ°јоТєЦ®З°Ј¬»№У¦ёГЅшРРµДІЩЧчКЗЈє_______________________ЎЈ
ЈЁ2Ј©µО¶ЁґпµЅЦХµгµДПЦПуКЗ______________________ЎЈ
ЈЁ3Ј©ёщѕЭЙПКцКэѕЭЈ¬їЙјЖЛгіцёГСОЛбµДЕЁ¶ИФјОЄ______________ЎЈЈЁ±ЈБфБЅО»УРР§КэЧЦЈ©
ЈЁ4Ј©іэИҐјоКЅµО¶Ё№ЬЦРЖшЕЭµД·Ѕ·ЁУ¦ІЙУГІЩЧч______________________Ј¬И»єуЗбЗбј·С№ІЈБ§ЗтК№јвЧмІї·ЦідВъјоТєЎЈЈЁМоРтєЕЈ©
ЈЁ5Ј©ФЪЙПКцКµСйЦРЈ¬ПВБРІЩЧчЈЁЖдЛыІЩЧчХэИ·Ј©»бФміЙІв¶ЁЅб№ыЖ«ёЯµДУР__________ЎЈЈЁ¶аСЎїЫ·ЦЈ©
AЈ®µО¶ЁЗ°ЖЅКУ¶БКйЈ¬ЦХµг¶БКэК±СцКУ¶БКэ
BЈ®ЛбКЅµО¶Ё№ЬК№УГЗ°Ј¬Л®ПґєуОґУГґэІвСОЛбИЬТєИуПґ
CЈ®Ч¶РОЖїЛ®ПґєуУЦУГ±кЧјСОЛбИуПґ
DЈ®іЖБїЗ°NaOH№ММеЦР»мУРNa2CO3№ММе
EЈ®ЕдЦЖєГµДNaOH±кЧјИЬТє±ЈґжІ»µ±Ј¬Ії·ЦУлїХЖшЦРµДCO2·ґУ¦ЙъіЙБЛNa2CO3
FЈ®јоКЅµО¶Ё№ЬјвЧмІї·ЦУРЖшЕЭЈ¬µО¶ЁєуПыК§
4ЎўјтґрМв ДіС§ЙъУыУГТСЦЄОпЦКµДБїЕЁ¶ИµДСОЛбАґІв¶ЁОґЦЄОпЦКµДБїЕЁ¶ИµДЗвСх»ЇДЖИЬТєК±Ј¬СЎФсјЧ»щіИЧчЦёКѕјБЈ®ЗлМоРґПВБРїХ°ЧЈє
ЈЁ1Ј©УГ±кЧјµДСОЛбИЬТєµО¶ЁґэІвµДЗвСх»ЇДЖИЬТєК±Ј¬ЧуКЦ°СОХЛбКЅµО¶Ё№ЬµД»оИыЈ¬УТКЦТЎ¶ЇЧ¶РОЖїЈ¬СЫѕ¦ЧўКУ______Ј®Ц±µЅТтјУИлТ»µОСОЛбєуЈ¬ИЬТєУЙ»ЖЙ«±дОЄіИЙ«Ј¬Іў______ОЄЦ№Ј®
ЈЁ2Ј©ПВБРІЩЧчЦРїЙДЬК№ЛщІвЗвСх»ЇДЖИЬТєµДЕЁ¶ИКэЦµЖ«µНµДКЗ______
ЈЁAЈ©ЛбКЅµО¶Ё№ЬОґУГ±кЧјСОЛбИЬТєИуПґѕНЦ±ЅУЧўИл±кЧјСОЛбИЬТє
ЈЁBЈ©µО¶ЁЗ°Кў·ЕЗвСх»ЇДЖИЬТєµДЧ¶РОЖїУГХфБуЛ®Пґѕ»єуГ»УРёЙФп
ЈЁCЈ©ЛбКЅµО¶Ё№ЬФЪµО¶ЁЗ°УРЖшЕЭЈ¬µО¶ЁєуЖшЕЭПыК§
ЈЁDЈ©¶БИЎСОЛбМе»эК±Ј¬їЄКјСцКУ¶БКэЈ¬µО¶ЁЅбКшК±ё©КУ¶БКэ
ЈЁ3Ј©ИфµО¶ЁїЄКјєНЅбКшК±Ј¬ЛбКЅµО¶Ё№ЬЦРµДТєГжИзНјЛщКѕЈє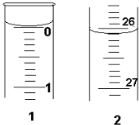
ФтЖрКј¶БКэОЄ______mLЈ¬ЦХµг¶БКэОЄ______mLЈ»ЛщУГСОЛбИЬТєµДМе»эОЄ______?mLЈ®
ЈЁ4Ј©ДіС§ЙъёщѕЭИэґОКµСй·Ц±рјЗВјУР№ШКэѕЭИз±нЈє
| µО¶ЁґОКэ | ґэІвNaOHИЬТєµДМе»э/mL | 0.1000mol/LСОЛбµДМе»э/mL µО¶ЁЗ°їМ¶И µО¶ЁєуїМ¶И ИЬТєМе»э/mL µЪТ»ґО 25.00 0.00 26.11 26.11 µЪ¶юґО 25.00 1.56 30.30 28.74 µЪИэґО 25.00 0.22 26.31 26.09 | ||
5ЎўМоїХМв КµСйКТУГNaOH№ММеЕдЦЖ0.1000mol?L-1 NaOHИЬТє500mLЈ®
ЈЁ1Ј©УГНРЕММмЖЅіЖИЎNaOH№ММе______gЈ®ИЬТєЕдЦЖ№эіМУГµЅПВБРІЈБ§ТЗЖчЈ¬°ґКЧґОК№УГµДПИєуЛіРтТАґОКЗ______ЈЁМоТЗЖчСЎПо·ыєЕЈ©Ј®
AЈ®ІЈБ§°фBЈ®ЅєН·µО№ЬCЈ®ЙХ±DЈ®500mLИЭБїЖї
ЈЁ2Ј©УГЛщЕдЦЖµД0.1000mol?L-1 NaOHИЬТєНЁ№эЦРєНµО¶ЁІв¶ЁТ»ФЄИхЛбHAИЬТєЕЁ¶ИЈ¬ГїґОµО¶ЁИЎУГµДHAИЬТєѕщОЄ20.00mLЈ¬К№УГ·УМЄИЬТєОЄЦёКѕјБЈ¬µО¶ЁЦХµгµД±кЦѕКЗ______Ј®µО¶ЁµДКµСйКэѕЭјЗВјЈє
| µО¶ЁґОКэ | NaOHИЬТєМе»эЈЁmLЈ© V1 V2 1 3.05 44 2 1.45 41.5 3 7.65 47.6 | |